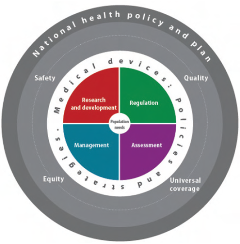
 Figure 1. The digram shows the relationship between the medical devices agenda and the interaction of successful functionality of each phase on the total expected outcomes of safety, quality, universal coverage, and equity. Source: WHO medical device technical series 2011. Click for a larger view The main goal of the health and biomedical devices programme is to ensure that medical devices produce maximum public health benefits at affordable costs in all intended settings.
Figure 1. The digram shows the relationship between the medical devices agenda and the interaction of successful functionality of each phase on the total expected outcomes of safety, quality, universal coverage, and equity. Source: WHO medical device technical series 2011. Click for a larger view The main goal of the health and biomedical devices programme is to ensure that medical devices produce maximum public health benefits at affordable costs in all intended settings.
The overarching values of universality, access to good quality care, equity and safety constitute a set of values that are shared across the Region. The programme's strategy is structured on these values and relies mainly on five major crucial components, which are commonly known as the five A's – availability, accessibility, appropriateness, affordability and accountability.
Availability: enhancing capacity of Member States to adequately assess and prioritize their technology needs, thereby making essential technologies available to the public.
Accessibility: improving equitable access to safe, high quality, reliable and adequate technologies and clinical services.
Appropriateness: promoting medical methods, procedures, techniques, and equipment that are scientifically valid, adapted to local needs, acceptable to both patient and the health care personnel, and that can be utilized and maintained using affordable resources.
Affordability: ensuring that medical devices produce maximum public health benefits at affordable costs in all settings.
Accountability: strengthening regulatory authorities for medical devices by making professionals, governments and companies accountable for ensuring safety, quality and efficacy of the used device and/or technology.
A regional consultation on medical devices will be held in the second half of 2012 for the purpose of developing the regional strategy on medical devices based on available regional data and baseline country surveys conducted during the last biennium.
Related links
Eastern Mediterranean regional strategy for appropriate health technology [pdf 792kb]






