A.A. Gunaid1 and A.M. Assabri2
انتشار النمط الثاني من السكري وسائر عوامل الاختطار القلبية الوعائية في منطقة شبه ريفية في اليمن
عبد الله أحمد جنيد، وعلي محمد الصبري
الخلاصـة: حدَّدت هذه الدراسة مدى انتشار النمط الثاني من السكري، وخلل تحمل الغلوكوز، وسائر عوامل اختطار الأمراض القلبية الوعائية، وذلك في عينة قوامها 250 من البالغين الذين هم في عمر 35 عاماً أو أكثر، في منطقة شبه ريفية قريبة من العاصمة اليمنية صنعاء. وبلغ معدل الانتشار الإجمالي الخامّ للسكري 10.4% (عند فاصلة ثقة 95% مُتَراوحاً بين 6.6% و14.2%)، وبلغ معدل الانتشار بحسب العمر 6.3% (عند فاصلة ثقة 95% مُتَـراوحاً بين 5.4% و7.2%). وكان معدل الإصابة بخَلَل مقدار الغلوكوز على الريق أو بخلَلَ تحمل الغلوكوز، بحسب العمر، 9% (عند فاصلة ثقة 95% مُتَراوحاً بين 6% و12%)، وكان معدل انتشار فرط ضغط الدم بحسب العمر 14.2% (عند فاصلة ثقة 95% مُتَراوحاً بين 13% و16%). ولوحظت علاقة بين كل من العمر ومحيط الخصر وبين عدم التحمل الكلي للغلوكوز. ويشير ارتفاع التكرار الملاحظ للسمنة المركزية إلى وجود منشأ غذائي لهذه الحالات الصحية الضائرة.
ABSTRACT: The study determined the prevalence of type 2 diabetes, abnormal glucose tolerance and other cardiovascular risk factors in a sample of 250 adults aged ≥ 35 years in a semirural area near Sana’a, the capital of Yemen,. The overall crude prevalence of diabetes was 10.4% (95% CI: 6.6%–14.2%) and the age-standardized rate was 6.3% (95% CI: 5.4%–7.2%). The age-standardized rate of having either impaired fasting glucose or impaired glucose tolerance was 9.0% (95% CI: 6.0%–12.0%) and the age-standardized prevalence of hypertension was 14.2% (95% CI: 13.0%–16.0%). Age and waist circumference were independently related to total glucose intolerance. The observed high frequency of central obesity suggests a dietary origin for these adverse health conditions.
Prévalence du diabète de type 2 et des autres facteurs de risque cardio-vasculaire en zone semi‑rurale au Yémen
RÉSUMÉ: L’étude a déterminé la prévalence du diabète de type 2, de l’abaissement de la tolérance au glucose et des autres facteurs de risque cardio-vasculaire au sein d’un échantillon de 250 adultes âgés de 35 ans et plus résidant en zone semi‑rurale près de Sanaa, la capitale du Yémen. La prévalence brute globale du diabète était de 10,4 % (IC95 % : 6,6‑14,2 %) et le taux normalisé selon l’âge de 6,3 % (IC95 % : 5,4‑7,2 %). Le taux normalisé selon l’âge de l’intolérance au glucose à jeun et de l’abaissement de la tolérance au glucose a été établi à 9,0 % (IC95 % : 6,0‑12 %), tandis qu’en ce qui concerne l’hypertension artérielle la prévalence normalisée selon l’âge était de 14,2 % (IC95 % : 13,0‑16,0 %). Il est apparu une relation indépendante entre l’âge, d’une part, et le tour de taille, d’autre part, et l’intolérance au glucose totale. La fréquence élevée de l’obésité centrale que l’on a pu observer évoque une origine alimentaire pour ces pathologies.
1Department of Medicine; 2Community Medicine, Faculty of Medicine and Health Sciences, University of Sana’a, Sana’a, Yemen (Correspondence to A.A. Gunaid:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
Received: 11/09/05; accepted: 19/12/05
EMHJ, 2008, 14(1): 42-56
Introduction
Type 2 diabetes mellitus is the epidemic of the new millennium [1]. An apparent epidemic of diabetes has occurred in the adult population throughout the world. At least 100 million people today suffer from type 2 diabetes, but by 2010, 215 million people are projected to have the disease [2]. It is the populations in developing countries and the minority or disadvantaged communities in the developed countries who now face the greatest risk [3]. This phenomenon is believed to be associated with the drastic change in dietary habits that accompanies either the modernization of these traditional populations or migration to developed countries where diets are of the Western type [4,5]. This finding was explained by the observation that the diets in industrialized countries have a “high energy density” compared with traditional ones. It was also proposed that with modernization and migration, traditional populations consume dietary sucrose in genetically unknown forms and dietary fat in genetically unknown quantities thus leading to harmful metabolic consequences, including obesity and type 2 diabetes [5].
The prevalence of type 2 diabetes mellitus in a country can rise over the relatively short period of 1 to 2 decades as more people become urbanized [6]. Epidemiological studies have shown that major risk factors for type 2 diabetes mellitus include increasing age, greater obesity, unfavourable fat distribution, physical inactivity, excess dietary fat, hyperinsulinaemia and genetic susceptibility [7].
In the countries of the Eastern Mediterranean Region, socioeconomic development and changes of lifestyle have been accompanied by the emergence of diabetes as a major health problem. For type 2 diabetes in adults, the risk is higher in urban than in rural people, and in all populations the prevalence increases with advancing age [8].
In Yemen, our knowledge about the epidemiology of diabetes mellitus remains poor, and there is little information available on this issue in the international literature. Thus, the aim of our study was to determine the prevalence of type 2 diabetes mellitus, impaired glucose tolerance (IGT), impaired fasting glucose (IFG) and other cardiovascular risk factors among the adult population in a semirural area near the capital city, Sana’a.
Methods
The semirural area of Hamdan, approximately 20 km from Sana’a was chosen for the survey. This mountainous area consists of 4 districts including 16 small villages with a total population of 70 478 inhabitants. Residents aged ≥ 35 years were estimated to account for 33% of the total population according to the 1994 census [9]. Health services in this area are provided by 1 health centre.
Sample
The sample size was calculated using Epi-Info, version 6.02, taking into consideration the following criteria: target population (men and women aged ≥ 35 years) = 23 258; expected frequency = 5.0% (no previous population-based study exists on diabetes in Yemen); worst acceptable result = 2.5%; sample size, with 95% confidence level = 288 people.
In order to secure a representative sample of the study population, a multistage random technique was used [10]. The names of eligible people by sex from the randomly selected households were registered in a list which was used for preparing invitations to the survey and for monitoring the response. People were invited to attend on a specific day during a 3-week period in August 2000.
Data collection
The survey team comprised medically qualified personnel who were carefully trained how to conduct the fieldwork and were supervised by the authors. The participants arrived in the morning (08:00–09:00 hours) after an overnight fast. After registration, the medical history was taken and a clinical examination and blood test were carried out. We used the World Health Organization (WHO) standard field guide for diabetes and noncommunicable disease risk factor surveys [11].
Demographic data. Demographic data collected included name, sex, age, address, level of education and occupation of head of household. Social class classification, based on occupation of the head of household, included administrative/professional (social class 1), business (social class 2), skilled labour (social class 3), manual labour (social class 4 and 5) unemployed and pensioner.
Medical history. Medical history consisted of history of hypertension and diabetes in the index subject and/or first-degree relatives, consanguinity of parents, occupational and leisure physical activity and smoking habit. Obstetric history in women included the number of babies born alive or dead and number of miscarriages.
Clinical data. Clinical examination included height and weight measurement and calculation of body mass index (BMI), waist and hip measurement and calculation of waist-to-hip ratio (WHR), and measurement of systolic and diastolic blood pressure (BP). Height was measured without shoes with the person standing erect on a flat surface and it was recorded to the nearest centimetre. Weight was measured with the person wearing light clothing and recorded to the nearest 0.1 kg. Waist circumference was measured twice using a special tape and averaged to the nearest centimetre with the person standing. The measurement was made half way between the lower border of the ribs and the iliac crest, normally with the tape horizontal. Hip circumference was measured twice using the same tape and was averaged to the nearest centimetre. Measurement was made at the maximum circumference, which is normally at the level of the greater trochanter with the person standing sideways to the investigator.
BMI (kg/m2) was calculated and classified as follows [11]: normal (BMI 18.5–24.9 kg/m2), grade 1 overweight (BMI 25.0–29.9 kg/m2), grade 2 overweight (30.0–39.9 kg/m2) and grade 3 overweight (BMI ≥ 40 kg/m2). The circumferences of waist and hip were used for calculation of the WHR. Central obesity was defined as a WHR ≥ 0.85 for women and ≥ 0.95 for men [11]. High-risk abdominal adiposity was also defined as a waist circumference ≥ 88 cm for women and ≥ 102 cm for men [12].
BP was measured and evaluated using a mercury sphygmomanometer and a standard clinical protocol according to the JNC-VI report [13]. Two readings of the systolic and diastolic BP separated by 2 minutes were averaged to the nearest 2 mmHg from the top of the mercury meniscus. Systolic BP was recorded at the first appearance of sounds, and diastolic BP at phase V at the disappearance of sounds. Hypertension was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg. The validity of the weight scales and sphygmomanometers was ensured by calibration prior to their use.
The final step was measurement of the capillary whole blood glucose concentration in the fasting state and 2 hours after an oral glucose load of 75 g of anhydrous glucose dissolved in 250 mL water according to a standard protocol [14]. For those with a previous diagnosis of diabetes who were on regular treatment with oral hypoglycaemic agents or insulin, blood glucose was measured in the fasting state and 2 hours after breakfast. The capillary whole blood glucose was measured in mmol/L using a blood glucose meter, Super Glucocard GT-1640, and Glucocard Test Strip II (Arkray Inc., Kyoto, Japan). The validity of these glucometers was measured by comparing their readings with those of an autoanalyser (Alcyon 300, Abbott Laboratories, USA). Using the current WHO criteria [14], diabetes mellitus was defined as 2-hour capillary whole blood glucose concentration ≥ 11.1 mmol/L, and IGT as a fasting capillary whole blood glucose concentration < 6.1 mmol/L and 2-hour capillary whole blood glucose concentration from ≥ 7.8 mmol/L to < 11.1 mmol/L. IFG was defined according to the recently introduced American Diabetes Association (ADA) criteria [15] as a fasting capillary whole blood glucose concentration from ≥ 5.6 mmol/L to < 6.1 mmol/L, and 2-hour capillary whole blood glucose concentration < 7.8 mmol/L.
Analysis
Data were collected in a personal computer and statistical analysis was conducted using 3 statistical software packages: SPSS, version 9.00, Epi-Info, version 6.02, and Confidence interval analysis (CIA), version 1.0 [16].
The prevalence rates and 95% confidence interval (CI) of diabetes, IGT, IFG, either IFG or IGT, and hypertension for the age range 30–64 years of the population in Yemen were calculated using the world population as the standard [17,18]. According to this method, the prevalence rates were standardized for age in a truncated age range of 30–64 years using 10-year age groups and the Yemen standard population for the year 2000. An epidemicity index of diabetes in the study population was also calculated according to the method described by Dowse et al. [19] as the percentage of total glucose intolerance (TGI) made up by IFG/IGT, i.e. (IFG/IGT × 100)/TGI.
Continuous variables were expressed as means [standard deviation (SD)] and a 2-tailed t-test was used for calculating statistical significance. Multiple linear regression analysis was computed using the SPSS package to indicate the predictors of blood glucose and BP values.
Univariate and stepwise multivariate logistic regression analysis were also computed using the SPSS package to define the odds ratio (OR) of risk factors independently related to glucose intolerance. The CIA software package was used to calculate the 95% CI in order to indicate precision of sample estimate, the variability of the characteristics being studied and the degree of confidence required. P < 0.05 was taken as statistically significant.
Results
A total of 288 people aged ≥ 35 years were invited to participate in the survey. Females represented 52% of the total sample. The number of people responding was 250, representing an overall participation rate of 86.8%.
The median age of the study sample was 50 years (range 35–90 years). The mean age was 55.9 (SD 14.9) years for men and 46.8 (SD 10.2) years for women. The baseline characteristics of the study sample (data not shown) indicated that 50% of men and 89% of women were illiterate; and that 85.6% of the sample belonged to social class 4 and 5 combined (nonskilled manual labour) and 7.6% to social class 3 (skilled labour). Current smoking was reported by 36.6% of men and 11.8% of women. About 18% had first or second cousin consanguineous parents. A family history of diabetes and hypertension among first-degree relatives was reported only by 5.2% and 6.0% of the study sample respectively. However, the frequency of family history of diabetes among the first-degree relatives of participants with TGI was significantly higher than among the relatives of those with normal glucose tolerance (12.7% versus 2.7%, OR = 5.3, 95% CI: 1.5%–19.6%, χ2 = 9.6, P = 0.002).
Crude and age-standardized prevalence rates of diabetes, IGT, IFG, and either IFG or IGT are shown in Table 1. The overall crude prevalence of diagnosed and undiagnosed diabetes was 10.4% (95% CI: 6.6%–14.2%) with a slightly higher rate in women (11.0%) than in men (9.8%). Among those with diabetes, 50% of the men and 57% of the women were newly diagnosed. The age-standardized prevalence of diabetes for age range 30–64 years was 6.3% (95% CI: 5.4%–7.2%) with a higher rate in women 8.6% (95% CI: 7.4%–9.8%) than in men 3.5% (95% CI: 2.6%–4.4%). In general, the prevalence of diabetes (either diagnosed or undiagnosed) increased with age.
The overall crude prevalence of IGT was 11.2% (95% CI: 7.3%–15.0%) with a slightly higher rate in men (13.0%) than in women (9.5%) (Table 1). The age-standardized prevalence of IGT for age range 30–64 years was 6.0% (95% CI: 5.0%–7.0%) with a higher rate in women 6.8% (95% CI: 5.5%–8.0%) than in men 4.6% (95% CI: 3.1%–6.1%). The overall crude prevalence rate of IFG was 3.6% (95% CI: 1.7%–6.7%) with a higher prevalence in women (5.5%) than in men (1.6%). The age-standardized prevalence rate of IFG for age group 30–64 years was 2.8% (95% CI: 1.9%–3.8%) with a higher rate in women 4.2% (95% CI: 3.3%–5.0%) than in men 1.0% (95% CI: 0.0%–1.6%).
The overall crude prevalence of people having either IFG/IGT was 14.8% (95% CI: 10.4%–19.2%) with a slightly higher rate in women (15.0%) than in men (14.6%) (Table 1). The age-standardized prevalence rate of either IFG/IGT for age range 30–64 years was 9.0% (95% CI: 6.0%–12.0%) with a higher rate in women 10.9% (95% CI: 7.1%–14.7%) than in men 5.7% (95% CI: 2.8%–8.6%). In general, the prevalence of either IFG/IGT increased with age.
The overall crude prevalence of TGI (diabetes, IFG and IGT combined) was 25.2% (95% CI: 19.8%–30.6%) with a slightly higher rate in women 26.0% (95% CI: 18.4%–33.6%) than in men 24.4% (95% CI: 16.8%–32.0%). The age-standardized prevalence of TGI for age range 30–64 years was 19.5% (95% CI: 17.8%–21.2%) for women and 9.2% (95% CI: 8.1%–10.3%) for men.
The prevalence of clinical hypertension is shown in Table 2. The overall crude prevalence of diagnosed and undiagnosed hypertension was 26.0% (95% CI: 21.0%–31.4%) with a higher rate in men (29.3%) than in women (22.8%). Among those with hypertension, 75% of the men and 62% of the women were newly diagnosed. The age-standardized prevalence rate of clinical hypertension for age range 30–64 years was 14.2% (95% CI: 13.0%–16.0%) with a slightly higher rate in women 14.8% (95% CI: 13.4%–16.3%) than in men 13.3% (95% CI: 12.3%–14.4%). In general, the prevalence of clinical hypertension (either diagnosed or undiagnosed) increased with age.
A total of 12 people in the survey had a prior diagnosis of diabetes confirmed in the records. Of these, 8 people were treated with oral hypoglycaemic agents, 2 used insulin and the remaining 2 claimed to use herbal remedies. In addition, 20 people had a prior record-based diagnosis of hypertension; of these, 14 (70.0%) were receiving treatment. Current intake of medications for diabetes or hypertension was verified by the survey team.
The epidemicity index of diabetes in the study population was also calculated as the percentage of TGI composed of IFG/IGT. Out of 63 people with total glucose intolerance, 37 had IFG/IGT, corresponding to an epidemicity index of 58.7%, i.e. there was a high prevalence of IFG/IGT in the presence of a low prevalence of diabetes in the given population.
The relationship between glucose tolerance status and some selected demographic and biomedical factors of the population is presented in Table 3. In men, the mean values of age, WHR, waist circumference and BP were generally higher in people with IFG/IGT and diabetes than in those with normal glucose tolerance. Although a greater number of men with normal glucose tolerance or with IFG/IGT claimed to be habitually more physically active than those with diabetes, the difference was not statistically significant. About 27% of men were overweight (BMI ≥ 25 kg/m2) and nearly 42% had central obesity (WHR ≥ 0.95). In women, the mean values of age, BMI, WHR, waist circumference and BP were generally higher in women with IFG/IGT and diabetes than in those with normal glucose tolerance. More than half of the women in the study claimed to be habitually physically active and there was no statistical trend in this variable. More than one-third of the women were overweight (BMI ≥ 25 kg/m2) and about two-thirds had central obesity (WHR ≥ 0.85).
Multiple linear regression analysis was computed with the aim of predicting participants’ capillary blood glucose concentration and BP values on the basis of the variables studied. Mean values of fasting blood glucose, 2-hour blood glucose, systolic BP and diastolic BP were used as dependent variables, and age, BMI, waist circumference and WHR values as independent variables. No consistent association between WHR and each of blood glucose and BP variables was observed (data not shown). Age elicited a significant positive correlation to the mean values of both fasting blood glucose (regression coefficient β = 0.027, P = 0.005) and 2-hour blood glucose (β = 4.66, P = 0.002), and to the mean systolic BP value (β = 0.505, P < 0.0001). BMI was a significant predictor of the mean values of both systolic BP (β = 1.28, P < 0.0001) and diastolic BP (β = 0.821, P < 0.0001) but not of mean blood glucose concentrations. In contrast, waist circumference was a significant predictor of the mean values of both fasting blood glucose (β = 0.033, P = 0.004) and 2-hour blood glucose (β = 6.34, P = 0.001) but not of BP variables.
The adjusted ORs of TGI (diabetes, IFG and IGT combined) for a number of variables were computed using the stepwise multivariate logistic regression analysis (Table 4). Factors selected in the model were age ≥ 55 years, male sex, family history of diabetes, overweight (BMI ≥ 25 kg/m2), WHR (≥ 0.95 for men and ≥ 0.85 for women), waist circumference (≥ 102 cm for men and ≥ 88 cm for women), hypertension (BP ≥ 140/90 mmHg), current smoking, social class and physical inactivity (data not shown).
Separate analysis was performed for men and women to detect any sex differences in risk factors for glucose intolerance. No such differences were observed. Male sex, family history of diabetes, BMI, WHR, current smoking and social class were not independently related to glucose intolerance and were therefore excluded from the model by the stepwise procedure. The risk factors independently related to TGI were only age ≥ 55 years (OR = 3.34, 95% CI: 1.78–6.25) and waist circumference (≥ 102 cm for men and ≥ 88 cm for women) (OR = 2.35, 95% CI: 1.21–4.58). Family history of diabetes among first-degree relatives, which was statistically significant in univariate analysis, was no longer a predictor of TGI on the multivariate logistic regression model.
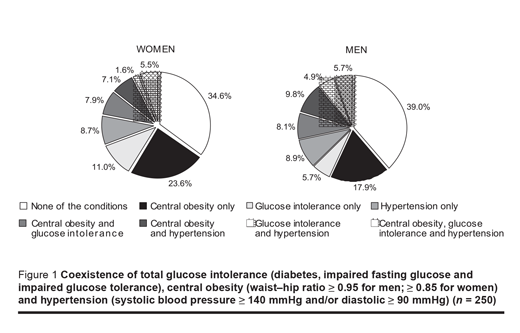
The coexistence of TGI, central obesity and hypertension in survey responders was also investigated (Figure 1). One or more of these conditions was present in 61.0% of men and in 65.4% of women. The most frequent condition was central obesity, being present alone in 18.0% of men and in 23.6% of women, and being complicated by glucose intolerance in 8.0% of each men and women, or by hypertension in 9.8% of men and 7.0% of women. Isolated cases of glucose intolerance were found in 5.7% of men and 11.0% of women. Hypertension alone was found in 8.9% of men and 8.7% of women. The combination of glucose intolerance and hypertension was observed in 4.9% of men and in only 1.6% of women. All 3 conditions combined were demonstrated in a further 5.7% of men and 5.5% of women.
Discussion
This population-based survey was conducted on adults aged ≥ 35 years living in a semirural area in Yemen. The overall crude prevalences of diabetes, either IFG/IGT and hypertension were 12.8%, 11.2% and 26.0% respectively. The age-standardized prevalence rate for the age range 30–64 years in this group in Yemen was found to be 6.3% for diabetes, 9.0% for either IFG/IGT, 6.0% for IGT alone and 14.2% for hypertension. Both diabetes and either IFG/IGT were slightly more frequent in women than in men, and both were higher in people aged ≥ 65 years than those aged 35–64 years. In contrast, in a well-defined urban community in Yemen the age-standardized prevalence of self-reported (diagnosed) diabetes among adults aged 30–64 years was 9.75% (95% CI: 7.55%–11.95%) [20]. We can assume that this figure could be doubled if the estimate was based on measuring blood glucose 2 hours after a standard oral glucose load [21]. The age-standardized prevalence of diabetes (either previously diagnosed or newly diagnosed) was even higher, amounting to 25.5% (95% CI: 17.4%–33.7%) among adult Yemeni immigrants in the United States [22].
Using the WHO diagnostic criteria, several population-based studies have been recently published indicating the prevalence of diabetes and IGT in semiurban and rural communities in Eastern Mediterranean Region countries. Table 5 shows the prevalence of diabetes and IGT in rural and semirural communities in Sudan [23], Jordan [24], Egypt [25,26], Lebanon [27], Saudi Arabia [28] and Tunisia [29]. Although the WHO diagnostic criteria were adopted by the great majority of these studies, there were some differences in the age groups studied and the prevalence rates were not age-standardized. This may explain the diversity in the prevalence of diabetes and IGT among rural and semirural adult populations in the Region. For diabetes and IGT, comparability might be enhanced if comparable methodology and diagnostic criteria were employed [30] and if the prevalence rates were standardized for age in a truncated age range of 30–64 years of the population studied [3,17,18].
The epidemicity index of diabetes in our study was relatively high. High prevalence of IFG/IGT in the presence of low prevalence of diabetes among the given semirural population might be taken as an indicator of the potential for a higher future prevalence of type 2 diabetes [19]. There is now some evidence that a single positive result of IGT carries a risk of developing type 2 diabetes of 2% to 8% over the following 5 to 10 years [31] and that the conversion from IGT to type 2 diabetes is associated with higher levels of plasma glucose within the IGT range, weight gain and greater obesity, hyperinsulinaemia and sedentary lifestyle [32,33]. The relatively high prevalence of IFG/IGT in our study might also be taken as an indicator of increased future risk of cardiovascular disease among this population, as was the case in the Whitehall study when the 2-hour blood glucose level exceeded 5.5 mmol/L, albeit using a 50 g glucose load [34].
Our data demonstrated that both ageing and central obesity were significantly associated with TGI in the study population. Coexistence of total glucose intolerance, central obesity and hypertension was obvious both in men and women, indicating clustering of these cardiovascular risk factors in the study population. Although diabetes was associated with advanced age in both sexes, the mean age difference between diabetes and normal glucose tolerance was considerably higher in men (8.4 years) than in women (3.4 years). In contrast, although diabetes was associated with central obesity in both sexes, the mean waist circumference difference between diabetes and normal glucose tolerance was slightly higher in women (7.9 cm) than in men (6.9 cm). Overweight was obviously more frequent in diabetes than in NGT especially in women, and hypertension was obviously more frequent in diabetes than in IGT especially in men. Finally, although diabetes was associated with a sedentary lifestyle in both sexes, the high rate of self-reported levels of physical activity casts doubts on the validity of our activity scale.
The significance of age and waist circumference, but not of BMI, in predicting people’s capillary whole blood glucose concentration in the multiple regression model confirms that age and central obesity, rather than body mass, are the factors of importance. This association was further confirmed by the finding that age ≥ 55 years and waist circumference (≥ 102 cm in men and ≥ 88 cm in women) were independently related to TGI (diabetes, IFG and IGT combined) on the multivariate stepwise logistic regression model. However, the contribution of genetic susceptibility to the prevalence of diabetes was not evident in this population.
Clinical hypertension in our study was particularly high by regional standards, where at least 20% of the population aged 20 years or older in the WHO Eastern Mediterranean countries suffers from hypertension [35]. The proportion of subjects with previously diagnosed hypertension was notably low in our area (8%) and this probably represents a lack of regular screening programmes in Yemen.
In summary, the results obtained from this study indicate that diabetes and other cardiovascular risk factors are already a significant public health problem in this sample of the adult population in Yemen, with the prevalence increasing with advancing age. However, this small semirural area is but one of the many and varied regions of the country and further studies would be required to obtain a complete picture of the population distribution of diabetes mellitus and other cardiovascular risk factors in Yemen. Bearing in mind the limited contribution of genetic factors, the high frequency of central obesity suggests a dietary origin of these adverse health conditions. Thus, we recommend establishing a community awareness programme highlighting the consequences of these public health problems and the usefulness of a healthy diet and adequate exercise.
Acknowledgements
We are very grateful to the survey team for completing the field work efficiently and on time: Abdo M.S. Al-Assad, Abdell-Basat M.M. Al-Kamaly, Adel H. Al-Shaikh, Ali A.A. Al-Kureimi, Khalid F.M. Saleh, Mohammed H.M. Al-Saidy. We also express our thanks to Mr Mohamed Al-Kubati for his statistical advice.
References
- Jovanovic L, Gondos B. Type 2 diabetes: the epidemic of the new millennium. Annals of clinical and laboratory medicine, 1999, 29(1):33–42.
- Zimmet PZ, McCarthy DJ, de Courten MP. The global epidemiology of non-insulin-dependent diabetes mellitus and the metabolic syndrome. Journal of diabetes and its complications, 1997, 11:60–8.
- King H, Rewers M. Diabetes in adults is now a Third World problem. World Health Organization ad hoc diabetes reporting group. Bulletin of the World Health Organization, 1991, 69(6):643–8.
- Zimmet P. Type 2 (non-insulin-dependent) diabetes—an epidemiological overview. Diabetologia, 1982, 22:399–411.
- Baschetti R. Diabetes epidemic in newly westernized populations: is it due to thrifty genes or to genetically unknown foods. Journal of the Royal Society of Medicine, 1998, 91:622–5.
- Vaughan P, Gilson L, Mills A. Diabetes in developing countries: its importance for public health. Health policy and planning, 1989, 4(2):97–109.
- \Harris M. Epidemiologic studies on the pathogenesis of non-insulin-dependent diabetes mellitus (NIDDM). Clinical and investigative medicine, 1995, 18(4):231–9.
- Alwan A, King H. Diabetes in the Eastern Mediterranean Region. World health statistics quarterly, 1992, 45(4):355–9.
- Central Statistical Organization: population and housing census (December 1994). Final results for Sana’a governorate, second report. Sana’a, Republic of Yemen, Ministry of Planning and Development, 1996:20, 31–2.
- Banker DJB, Hall AJ. Practical epidemiology, 4th ed. Edinburgh, Churchill Livingstone, 1991:40–3.
- King H. Diabetes and noncommunicable disease risk factor surveys—a field guide. Geneva, World Health Organization, 1999:51–61 (WHO/NCD/NCS/99.1).
- Bjorntorp P. Obesity. Lancet, 1997, 350:423–6.
- 13. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Archives of internal medicine. 1997, 157:2413–46.
- Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Definition, diagnosis, and classification of diabetes mellitus and its complications. Geneva, World Health Organization, 1999 (WHO/NCD/NCS/99.2).
- American Diabetes Association. Report of the Expert Committee on Diagnosis and Classification of Diabetes Mellitus. Diabetes care, 1997, 20:1183–97.
- Gardner MJ, Altman DG. Statistics with confidence: confidence intervals and statistical guidelines. London, British Medical Journal Publishing Group, 1997.
- King H, Rewers M. WHO Ad Hoc Diabetes Reporting Group. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes care, 1993, 16:157–77.
- World population prospects. The 1998 revision. Volume II: Sex and age distribution of the world population. New York, United Nations, Department of Economic and Social Affairs, Population Division, 1999:860–3.
- Dowse GK, Zimmet PZ, King H. Relationship between prevalence of impaired glucose tolerance and NIDDM in a population. Diabetes care, 1991, 14:968–74.
- Gunaid AA. Prevalence of known diabetes and hypertension in the Republic of Yemen. Eastern Mediterranean health journal, 2002, 8:374–85.
- Pinto NR, Franco LJ, Moncau JE. Comparación de cinco métodos para estimar la prevalencia de diabetes mellitus en estudios de base poblacional [Comparison of five methods to estimate the prevalence of diabetes mellitus in population-based studies]. Pan American journal of public health, 1997, 2:260–7.
- Jaber LA et al. Epidemiology of diabetes among Arab Americans. Diabetes care, 2003, 26:308–13.
- El-Bagir MN et al. Population-based study of the prevalence of diabetes and impaired glucose tolerance in adults in northern Sudan. Diabetes care, 1996, 19:1126–8.
- Ajlouni K, Jaddou H, Batieha A. Diabetes and impaired glucose tolerance in Jordan: prevalence and associated risk factors. Journal of internal medicine, 1998, 244:317–23.
- Herman WH et al. Diabetes mellitus in Egypt: risk factors and prevalence. Diabetic medicine, 1995, 12:1126–31.
- Herman WH et al. Diabetes mellitus in Egypt: risk factors, prevalence and future burden. Eastern Mediterranean health journal, 1997, 3(1):144–8.
- Salti IS et al. Epidemiology of diabetes mellitus in relation to other cardiovascular risk factors in Lebanon. Eastern Mediterranean health journal, 1997, 3(3):462–71.
- Al-Nuaim AR et al. National chronic metabolic diseases Survey. Part 1. Riyadh, Saudi Arabia, Ministry of Health/King Saud University, 1995:41–5.
- Papoz L et al. Diabetes mellitus in Tunisia: description in urban and rural populations. International journal of epidemiology, 1988, 17(2):419–21.
- Alwan A, King H. Diabetes in the Eastern Mediterranean (Middle East) Region: the World Health Organization responds to a major public health challenge. Diabetic medicine, 1995, 12:1057–8.
- Yudkin JS et al. Impaired glucose tolerance: is it a risk factor for diabetes or a diagnostic ratbag. British medical journal, 1990, 301:397–402.
- Alberti KGMM. Impaired glucose tolerance—fact or fiction. Diabetic medicine, 1996, 13:S6–8.
- Harris MI. Impaired glucose tolerance—prevalence and conversion to NIDDM. Diabetic medicine, 1996, 13:S9–11.
- Fuller JH et al. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. British medical journal, 1983, 287:867–70.
- Alwan A. Noncommunicable diseases: a major challenge to public health in the region. Eastern Mediterranean health journal, 1997, 3:6–16.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)