A. Gholami,1 S. Salarilak,2 S. Hejazi 3 and H.R. Khalkhali 4
الوزن عند الولادة واختطار ابيضاض الدم الحاد في الطفولة
علي غلامي، شاكر سالاري لك، ساسان حجازي، حميد رضا خلخالي
الخلاصـة: نظراً لأن الدراسات حول عوامل اختطار ابيضاض الدم الحاد مازالت غير جازمة فقد أجرى الباحثون هذه الدراسات للحالات والشواهد في ولاية غرب أذربيجان في جمهورية إيران الإسلامية للتعرُّف على العلاقة بين الوزن عند الولادة وابيضاض الدم الحاد لدى الأطفال دون سن الخامسة عشرة. وقد اختار الباحثون اثنين من الشواهد مقابل كل مريض يوافقانه بالعمر وبالجنس من السكان في المجتمع وفي المستشفى. وقد شملت الدراسة 130 مريضاً تم تشخيصهم خلال الفترة 2003–2009، وكان لدى 108 منهم (%83.1) نمط الأرومات اللمفاوية، ولدى 22 منهم (%16.9) نمط الأرومات النقوية. واتضح من حيث الاعتداد الإحصائي أن الذكور (%55.4) أكثر من الإناث (44.6). وعند إجراء النموذج التحوُّفي المتعدد المتغيِّرات اتضح أن المتغيرات التي تـترابط ترابُطاً يُعْتَدُّ به إحصائياً مع ابيضاض الدم الحاد هي: الوزن عند الولادة (نسبة الأرجحية 2.25)، الترتيب بين المولودين (نسبة الأرجحية 2.25)، مكان الولادة (نسبة الأرجحية 7.93)، قصة إصابة سابقة بالحُماق (نسبة الأرجحية 0.46)، درجة تعلُّم الأمهات (نسبة الأرجحية 3.23). واستنتج الباحثون أن خطر الابيضاض الحاد يزداد زيادة يُعْتَدُّ بها إحصائياً بازدياد الوزن عند الولادة في مجمل المجموعة وبين الفتيات لا بين الفتيان.
ABSTRACT Studies of risk factors for acute leukaemia are inconclusive. This case–control study was done in West Azerbaijan province, Islamic Republic of Iran, to determine the relationship between birth weight and acute leukaemia in children aged under 15 years. For every patient 2 age- and sex-matched controls were selected from hospital and community populations. Of 130 cases diagnosed over the period 2003–2009, 108 (83.1%) had lymphoblastic and 22 (16.9%) myloblastic type. Significantly more of them were male than female (55.4% versus 44.6%). In a multivariate logistic regression model variables significantly associated with acute leukaemia were: birth weight (OR = 2.25), birth order (OR = 2.25), birth place (OR = 7.93), history of chickenpox (OR = 0.46) and mothers’ education (OR = 3.23). The risk of acute leukaemia increased significantly with increasing birth weight in the total group and among girls, but not among boys.
Poids de naissance et risque de leucémie aiguë de l'enfant
RÉSUMÉ Les études des facteurs de risques d'une leucémie aiguë n'ont pas permis de tirer de conclusions. La présente étude cas-témoins a été menée dans la province d'Azerbaïdjan de l'ouest (République islamique d'Iran), pour déterminer la relation entre le poids de naissance et une leucémie aiguë chez l'enfant de moins de 15 ans. Pour chaque patient, deux témoins appariés pour l'âge et le sexe ont été sélectionnés dans des populations hospitalières ou communautaires. Sur 130 cas diagnostiqués entre 2003 et 2009, 108 (83,1 %) étaient porteurs du type lymphoblastique et 22 (16,9 %) du type myéloblastique. Les patients de sexe masculin étaient nettement plus nombreux que les patients de sexe féminin (55,4 % contre 44,6 %). Dans un modèle de régression logistique multivariée, les variables fortement associées à une leucémie aiguë étaient les suivantes : poids de naissance (OR = 2,25), rang de naissance (OR = 2,25), lieu de naissance (OR = 7,93), antécédents de varicelle (OR = 0,46) et niveau d'études de la mère (OR = 3,23). Le risque d'une leucémie aiguë augmentait nettement avec un poids de naissance accru dans l'ensemble du groupe et chez les filles, mais pas chez les garçons.
1School of Nursing, Neyshabur Faculty of Medical Sciences, Neyshabur, Islamic Republic of Iran.
2Department of Public Health, Islamic Azad University (Tabriz Branch), Tabriz, Islamic Republic of Iran (Correspondence to S. Salarilak:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
3Motahari Teaching Hospital, Children’s Blood Section; 4Department of Biostatistics and Epidemiology, Urmia University of Medical Sciences, Urmia, Islamic Republic of Iran.
Received: 21/11/11; accepted: 21/02/12
EMHJ, 2013, 19(2):156-161
Introduction
Leukaemia is a malignant progressive cancer which is caused by incomplete production of white blood cells and their precursors in blood and bone marrow. The disease is classified into acute and chronic types according to the speed of progression of cancer and into lymphoblastic or myeloblastic according to the types of white blood cell affected [1–3]. Leukaemia is the most prevalent childhood cancer and the most common type in children is acute lymphoblastic leukaemia (ALL). ALL is a very rapid cancer which is usually seen in ages between 2 and 6 years [2]. ALL has a high mortality rate, great costs for treatment and a long period of hospitalization, with associated psychological trauma for patients and their families [4].
In view of the social and economic burden of this disease, a number of studies have been done in different countries to study the relationship between genetic and environmental factors and the risk of acute leukaemia. One of these factors is birth weight and in some studies a statistically significant relationship has been reported between birth weight and leukaemia [5–21], while other studies have found no relationship [22–25]. In view of the conflicting findings on this subject, we decided to study the relationship between birth weight and acute leukaemia in children in the province of West Azerbaijan, Islamic Republic of Iran.
Methods
Study sample
This was a case–control study. The case group were patients with acute leukaemia, diagnosed from 20 March 2003 and 20 March 2009, age less than 15 years at the time of diagnosis and residing in West Azerbaijan province at the time of diagnosis. For each patient we selected 2 controls from 2 groups: a hospital control and a community control. Cases and controls were matched according to age group (< 5, 5–9 and 10–14 years old) and sex. Hospital controls were selected from the children’s ward and the children’s clinic centre in Motahari hospital in the city of Urmia. Community controls were selected from children who came to Urmia health centres for routine health care. Inclusion criteria for controls were not having acute leukaemia or any other blood disease, age less than 15 years at the time of data collection and residing in West Azerbaijan province.
Data collection
A specially designed questionnaire was used to collect data on the child’s demographic characteristics, type of cancer (for cases only), child’s risk factors (birth order, birth place, history of chickenpox, history of icterus, history of mumps infection, history of X-ray exposure, breastfeeding, formula-milk feeding) and parents’ demographic characteristics (mother’s age, father’s age, mother’s education and father’s education at the birth of the child). The questionnaire design was based on a questionnaire provided by the Washington Health Department [26] and questionnaires from previous studies in the Islamic Republic of Iran and with input from local professors of epidemiology and oncology. A pilot study was done to test the validity of the questionnaire. Questionnaires were completed using data files and face-to-face interviews with the mothers of patients and controls.
To prevent recall bias in the birth weight variable, we used the birth weight recorded in the household folders kept by the health system and in vaccination cards; the birth weight of 90.8% of the case patients and 95.0% of controls were recorded.
Statistical analysis
A logistic regression model in SPSS, version 16 was used to investigate the relationship between birth weight and acute leukaemia and also to control for confounding factors and investigate the possible effects on acute leukaemia risk of the risk factors investigated. We report odds ratios (OR), 95% confidence interval (CI) and P-values for all variables. The chi-squared test for trend was used to determine the trend of birth weight in the developing of acute leukaemia.
Results
A total of 138 children affected by acute leukaemia were diagnosed in west Azerbaijan province over the study period (113 with ALL and 25 with AML); 8 patients dropped out of the study, 1 because the parents did not agree to participation and 7 because of a change of address. So the study was based on 130 patients, of whom 108 (83.1%) were affected by ALL and 22 (16.9%) by AML. Of these, 72 patients (55.4%) were boys and 58 (44.6%) girls. At the time of diagnosis, 52.3% of their families lived in urban areas and 47.7% in rural areas. Most cases (43.1%) were aged under 5 years (Figure 1). There were 260 age and sex-match controls: 144 boys (55.4%) and 116 girls (44.6%).
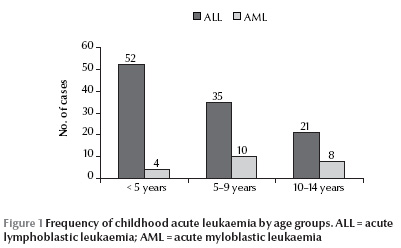
The mean birth weight of children affected by acute leukaemia [3400 (SD 650) g] was significantly higher than that the control group [3240 (SD 592) g] (P = 0.02).
A univariate logistic regression model was used to evaluate the contribution of each variable and its relation to leukaemia. Variables with a significant association were as follows: birth weight (< 4000 g versus ≥ 4000 g), birth order (1 versus ≥ 2), birth place (home versus hospital), history of chickenpox, mother’s and father’s age (< 35 versus ≥ 35 years) and mother’s and father’s education (below high school versus high school and above) (Table 1). Variables without any significant relationship according to the univariate logistic regression model were as follows: history of icterus, history of mumps, history of X-ray exposure, breastfeeding (≥ 6 months and < 6) and formula milk feeding (only formula feeding) (Table 1).
Using a multivariate logistic regression model with a forward method, we evaluated different variables in relation to acute leukaemia. Variables that remained significantly associated with acute leukaemia were as follows: birth weight (≥ 4000 versus < 4000 g) (OR = 2.25; 95% CI: 1.09–4.64), birth order (≥ 2 versus 1) (OR = 2.25; 95% CI: 1.33–3.83), birth place (home versus hospital) (OR = 7.93; 95% CI: 4.07–15.5), positive history of chickenpox (OR = 0.46; 95% CI: 0.25–0.96) and mothers’ education (below high school versus high school or above) (OR = 3.23; 95% CI: 1.77–5.89) (Table 2).
As seen in Table 3 , the risk of acute leukaemia increased with increasing birth weight category, from < 3000 g (OR = 0.65; 95% CI: 0.34–1.24), through 3000–3499 (reference group), 3500–3999 g (OR = 1.30; 95% CI: 0.76–2.20) to ≥ 4000 g (OR = 2.20; 95% CI: 1.09–4.50). This trend was statistically significant in the total group (P = 0.016) and among girls (P = 0.006), but not among boys (P = 0.133).
Discussion
In this study we examined 130 children with acute leukaemia who were diagnosed in West Azerbaijan province. In our sample of children diagnosed with acute leukaemia, 83.1% were affected by ALL and 16.9% by AML. In MacArthur et al.’s study in Canada 88% of patients were affected by ALL, 10% by AML and 2% by acute unknown lymphoblastic leukaemia [27]. In Milne et al.’s study in Western Australia 86.8% of children were affected by ALL and 13.2% by AML [28]. In all these studies, we see that more than 80% of acute leukaemia cases were ALL type.
Slightly more of the cases were among males than females (55.4% versus 44.6%). This agrees with the results of other research. For example, Hjalgrim et al. found 52.9% of patients were males and 47.1% were females [5], Rosenbaum et al., studying patients with ALL only, found 57% were males and 43% were females [29], while Mallol-Mesnard et al. reported that 54.4% of patients were males and 45.6% were females [30]. Most of the cases in our study were under 5 years of age (43.1%), which is similar to the results of Westergard et al. [7] Hjalgrim et al. [5] and Zolala et al. [22]. In our study the number of patients living in urban areas (52.3%) was greater more than in rural areas (47.7%). In Auvinen et al.’s study on ALL patients 24% of patients lived in rural areas, 32% in urban areas and 44% in suburban areas [31]. Zolala et al. reported 63.5% of patients living in urban areas and 36.5% in rural areas [22].
One of the major advantages of this study was that the subjects in the control group were randomly selected from the children’s ward and from the children’s clinic centre in Motahari hospital in Urmia city and from children who came to Urmia health centres for routine care rather than from recruited volunteers as in most other case–control studies. In addition, the use of vital records eliminated the problem of recall bias (in the birth weight variable) because the data were not self-reported after a diagnosis of leukaemia.
We observed a strong relationship between increasing birth weight and acute leukaemia risk. Several studies have suggested a possible relationship between acute leukaemia risk in children and particularly high birth weight [5–21,23,32–36]. For example in Hjalgrim et al.’s study a significant relationship was seen between birth weight and ALL but not between birth weight and AML [5]. In Caughey and Michel’s meta-analysis [6] and a meta-analysis by Hjalgrim et al [36], a significant relationship was seen between weight ≥ 4000 g at the time of birth and acute leukaemia (AML and ALL) [6,27]. Some studies did not observe any relationship between high birth weight and leukaemia [22–25].
We found a relationship not only for those with high birth weight but also a steady increase in leukaemia risk with increasing birth weight category. This relationship was statistically significant for girls but not for boys. This agrees with Westergard et al., who observed that for each kilogram increase in birth weight the relative risk increased by 1.46 in ALL and 2.14 in AML [7]. Robert et al. also observed that increasing birth weight increased the risk of acute leukaemia in children and the association was statistically significant [8]. Our findings might imply that factors that are influential on fetal growth play an important role in the development of childhood leukaemia. It was previously suggested that children with a high birth weight may be more likely to be exposed to diagnostic radiation in utero or in the neonatal period and that this radiation exposure might explain some of the excess risk associated with high birth weight [16]. It has been suggested that high birth weight may result from high levels of growth factors in uterus, and these growth factors might increase the risk of ALL by inducing proliferative stress on the bone marrow [37,38].
In conclusion, our study adds to the evidence that birth weight is related to an increased risk of childhood acute leukaemia and this may be useful for diagnosis and prevention. Further studies in Islamic Republic of Iran and other countries are needed to evaluate the risk factors for childhood leukaemia, including high birth weight.
Acknowledgements
We would like to extend best regards and thanks to the staff of the haematology ward in Motahari teaching hospital and also to the patient and control groups’ families.
Competing interests: None declared.
References
- Acute lymphocytic leukemia. Leukemia and Lymphoma Society [online factsheet] (http://www.lls.org/#/diseaseinformation/leukemia/acutelymphoblasticleukemia/ 2006, accessed 26 November 2012).
- Henry JB. Clinical diagnosis and management by laboratory methods, 20th ed. Philadelphia, Saunders, 2001.
- Acute myelogenous leukemia. Leukemia and Lymphoma Society [online factsheet] (http://www.lls.org/diseaseinformation/leukemia/acutemyeloidleukemia/2006, accessed 26 November 2012).
- Pizzo P. Poplack D. Principles and practice of pediatric oncology, 3rd ed. Philadelphia, Pennsylvania, Lippincott Williams and Wilkins, 2001:409–419.
- Hjalgrim LL et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. Journal of the National Cancer Institute, 2004, 96:1549–1556.
- Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. International Journal of Cancer, 2009, 124:2658–2670.
- Westergaard T et al. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. Journal of the National Cancer Institute, 1997, 89:939–947.
- Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. International Journal of Cancer, 2009, 124:2658–2670.
- Smith A et al.; UKCCS investigators. Birth weight, sex and childhood cancer: A report from the United Kingdom Childhood Cancer Study. Cancer Epidemiology, 2009, 33:363–367.
- Samuelsen SO et al. Birth weight and childhood cancer. Epidemiology, 2009, 20:484–487.
- McLaughlin CC et al. Birth weight, maternal weight and childhood leukaemia. British Journal of Cancer, 2006, 94:1738–1744.
- Schüz J, Forman MR. Birthweight by gestational age and childhood cancer. Cancer Causes and Control, 2007, 18:655–663.
- Kaye SA et al. Maternal reproductive history and birth characteristics in childhood acute lymphoblastic leukemia. Cancer, 1991, 68:1351–1355.
- Petridou E et al. The risk profile of childhood leukaemia in Greece: a nationwide case–control study. British Journal of Cancer, 1997, 76:1241–1247.
- Robison LL et al. Birth weight as a risk factor for childhood acute lymphoblastic leukemia. Pediatric Hematology and Oncology, 1987, 4:63–72.
- Daling JR et al. Birth weight and the incidence of childhood cancer. Journal of the National Cancer Institute, 1984, 72:1039–1041.
- Yeazel MW et al. High birth weight and risk of specific childhood cancers: a report from the Children’s Cancer Group. Journal of Pediatrics, 1997, 131:671–677.
- Schüz J et al. Association of childhood cancer with factors related to pregnancy and birth. International Journal of Epidemiology, 1999, 28:631–639.
- Ross JA et al. Evaluating the relationships among maternal reproductive history, birth characteristics, and infant leukemia: a report from the Children’s Cancer Group. Annals of Epidemiology, 1997, 7:172–179.
- Wertelecki W, Mantel N. Increased birth weight in leukemia. Pediatric Research, 1973, 7:132–138.
- Cnattingius S et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. Journal of the National Cancer Institute, 1995, 87:908–914.
- Zolala F et al. Determination the inducing factors of acute lymphoblastic leukemia in children under 15 years old in Fars province (Iran) in the year 2001. Rafsanjan of Medical Sciences and Health Services, 2004, 3:267–275.
- Eisenberg DE, Sorahan T. Birth weight and childhood cancer deaths. Journal of the National Cancer Institute, 1987, 78:1095–1100.
- Shu XO et al. Breast-feeding and risk of childhood acute leukemia. Journal of the National Cancer Institute, 1999, 91:1765–1772.
- Berger R. Acute lymphoblastic leukemia and chromosome 21. Cancer Genetics and Cytogenetics, 1997, 94:8–12.
- A survey related to childhood acute lymphocytic leukaemia. Whatcom County, Washington, Washington State Department of Health, 1999 (www.co.whatcom.wa.us/health/pdf/leukemia_survey.PDF, accessed 10 December 2012).
- MacArthur AC et al. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes and Control, 2008, 19:283–295.
- Milne E et al. Fetal growth and acute childhood leukemia: looking beyond birth weight. American Journal of Epidemiology, 2007, 166:151–159.
- Rosenbaum PF, Buck GM, Brecher ML. Early child-care and preschool experiences and the risk of childhood acute lymphoblastic leukemia. American Journal of Epidemiology, 2000, 152:1136–1144.
- Mallol-Mesnard N et al. Vaccination and the risk of childhood acute leukaemia: the ESCALE study (SFCE). International Journal of Epidemiology, 2007, 36:110–116.
- Auvinen A et al. Extremely low-frequency magnetic fields and childhood acute lymphoblastic leukemia: an exploratory analysis of alternative exposure metrics. American Journal of Epidemiology, 2000, 152:20–31.
- Fasal E, Jackson EW, Klauber MR. Birth characteristics and leukemia in childhood. Journal of the National Cancer Institute, 1971, 47:501–509.
- Shu XO et al. A population-based case–control study of childhood leukemia in Shanghai. Cancer, 1988, 62:635–644.
- Cnattingius S et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. Journal of the National Cancer Institute, 1995, 87:908–914.
- Hirayama T. Descriptive and analytical epidemiology of childhood malignancy in Japan. In: Kobayashi N, ed. Recent advances in management of children with cancer. Tokyo, The Children’s Cancer Association of Japan, 1980:27–43.
- Hjalgrim LL et al. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. American Journal of Epidemiology, 2003, 158:724–735.
- Ross JA et al. Big babies and infant leukemia: a role for insulin-like growth factor-1? Cancer Causes and Control, 1996, 7:553–559.
- Albanes D, Winick M. Are cell number and cell proliferation risk factors for cancer? Journal of the National Cancer Institute, 1988, 80:772–775.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)