Research article
P. Salameh 1 and B. Barbour1
طراز السمنة والسّكريّ المرتبط بها لدى المراهقين اللبنانيين: دراسة ارتيادية
باسكال سلامة، بيرناديت بربور
الخلاصـة: يتزايد السّكريّ المرتبط بالسمنة في جميع أرجاء العالم. وقد قيس مؤشر كتلة الجسم، وغلوكوز الدم الشُّعَيْريّ في هذه الدراسة، في عينة اختيرت عشوائياً من مراهقين من ثلاث مدارسة لبنانية خاصة. وتمّ تقييم السمنة وفقاً للأصواء التي حددتها فرقة العمل الدولية المعنيّة بالسمنة. وقد تبيَّن أنه من بين ثلاث مئة تلميذٍ، كان هناك %18.7 معرّضين لاختطار السمنة، و%3.0 مصابين بالسمنة. وبلغ مستوى غلوكوز الدم المأخوذ عشوائياً < 140 مغ/دل لدى %10.3 من التلاميذ. وفي عيّنات الدم المأخوذة على الريق، شوهد لدى %10.5 منهم عدم تحمّل للغلوكوز، في حين كان %3.5 منهم مصابين بالسكريّ. وكانت مستويات الغلوكوز أعلى على نحو يُعتدُّ به إحصائياً لدى الأفراد المفرطي الوزن مقابل الأفراد ذوي الوزن السَّوِيّ بمقدار: 86 (+ 13) مقابل 96 (+ 16) مع/دل. أما الأفراد الأسْوِيَاء الوزن فقد كان مستوى غلوكوز الدم غير سويّ في 8.6 منهم مقابل %37.0 من مفرطي الوزن كان غلوكوز الدم غير سويّ. وقد دلّت الدراسة على وجود معدلات مرتفعة من فرط الوزن ومن السّكريّ المرتبط بالسمنة ومن عدم تحمّل الغلوكوز لدى تلاميذ المدارس اللبنانيين.
ABSTRACT: Obesity-associated diabetes in adolescents is increasing throughout the world. In this study, body mass index and capillary blood glucose were measured in a randomly selected sample of adolescents from 3 Lebanese private schools. Obesity was evaluated according to International Obesity Task Force cut-offs. Out of 300 students, 18.7% were at risk of obesity and 3.0% were obese. Random glycaemia level was ≥ 140 mg/dL in 10.3% of students. In those fasting, 10.5% had glucose intolerance and 3.5% had diabetes. Glucose levels were significantly higher in overweight versus normal weight individuals: 86 (SD 13) versus 96 (SD 16) mg/dL. Among the normal weight group 8.6% had abnormal glycaemia while among those who were overweight 37.0% had abnormal glycaemia. Lebanese school students have high rates of overweight and of obesity-associated diabetes and glucose intolerance.
Obésité et diabète associé chez les adolescents libanais : une étude pilote
RÉSUMÉ: Le diabète associée à l’obésité chez les adolescents est en augmentation dans le monde. Dans la présente étude, l’indice de masse corporelle et la glycémie capillaire ont été mesurés dans un échantillon d’adolescents fréquentant trois écoles privées libanaises et sélectionnés au hasard. L’obésité a été évaluée à l’aide des valeurs seuils du Groupe de travail international sur l’obésité (IOTF). Sur 300 élèves, 18,7 % couraient un risque élevé d’obésité et 3,0 % étaient obèses. La glycémie aléatoire était supérieure ou égale à 140 mg/dl chez 10,3 % des élèves. Chez les élèves à jeun, 10,5 % souffraient d’une intolérance au glucose et 3,5 % étaient diabétiques. Les taux de glycémie étaient significativement supérieurs chez les enfants en surpoids par rapport aux enfants ayant un poids normal : 86 mg/dl (E.T. 13) par rapport à 96 mg/dl (E.T. 16). Dans le groupe d’enfants ayant un poids normal, 8,6 % présentaient un taux de glycémie anormal alors que ce taux était de 37 % chez les élèves en surpoids. Les élèves libanais présentent des taux élevés de surpoids, de diabète associé à l’obésité et d’intolérance au glucose.
1Faculty of Public Health, Lebanese University, Beirut, Lebanon (Correspondence to: P. Salameh: This e-mail address is being protected from spambots. You need JavaScript enabled to view it ). Received: 14/07/09; accepted: 07/09/09
EMHJ, 2011, 17(3): 226-230
Introduction
Childhood obesity is increasing worldwide and it has become the most important chronic disease of childhood [1]. Type 2 diabetes mellitus (DM) during childhood and adolescence, first diagnosed among Pima Indians in 1979, is accelerating in parallel [2–7]. In 2000, an alarming increase of childhood type 2 DM among ethnic minorities in the United States was described [2]. Type 2 DM has also been reported among children in Europe [4–6]. The evidence for an association between childhood obesity and type 2 DM is growing [2]. It has been demonstrated that severely obese children and adolescents with impaired glucose tolerance are at very high risk for developing type 2 DM over a short period of time [8].
Lebanon is a developing country in economic transition. However, epidemiological transition too is beginning to appear in the population. We can observe the gradual adoption of developed country lifestyles in terms of eating habits and physical inactivity. In previous studies, in both private [9,10] and public schools [11], Chakar and Salameh demonstrated that childhood obesity was a growing problem in Lebanon, In private schools, 10.1% of boys were obese and 28.8% at-risk of obesity (overweight but not yet obese) while 4.2% of girls were obese and 19.0% at-risk of obesity [9,10]. In public schools, as expected, lower rates were found for boys; 6.7% were obese and 20.8% at-risk of obesity, while for girls 6.0% were obese and 20.3% at-risk of obesity [11]. The objectives of this pilot study were to investigate the association between obesity and diabetes in Lebanese adolescents attending private schools.
Methods
Study population
This was a cross-sectional pilot study performed on adolescent school students attending Lebanese private schools between April and June 2007. School directors of 3 private schools located in an urban area of Mount Lebanon were contacted, and an informed consent form was sent to parents to be signed before the study began. Students aged 11–18 years of age were included in the study. A minimal sample size of 297 students was to be selected, given a prevalence of 5% and a precision of 2%. Out of the provided lists of students, a random sample of 300 students was selected from the 3 schools. Those reporting an existing diagnosis of DM were excluded and replaced by others.
Data collection
Short questionnaires were distributed to the students, and they were asked about their sex, age, diabetes-associated symptoms and if they had eaten anything on that day.
A fingerstick blood capillary sample was taken for glycaemia measurement (Accucheck) by 3 trained researchers in collaboration with the school health professional or school director. Random glycaemia (without necessarily fasting) was then classified as normal (< 140 mg/dL) or higher than normal (≥ 140 mg/dL), according to the recommendations of Rolka et al. for diabetes screening [12]. Fasting glucose was classified as follows: normal (< 100 mg/dL), prediabetes/glucose intolerance (100–125 mg/dL) or diabetes (≥ 125 mg/dL) [13].
Students’ weight and height were measured using the same calibrated balance (Soehle, sensitivity 500g) and stadiometer for height measurement (Stanley, MABO Microtorse); shoes were removed and measurements were made with light indoor clothing only. Body mass index (BMI) was calculated in kg/m2 and used to as a measure of obesity [14,15]. As adult cut-off values are not valid for adolescents [16], obesity and at-risk of obesity (i.e. overweight but not yet obese) were defined according to the cut-offs of the International Obesity Taskforce criteria for BMI of children aged 2–18 years, which for age 18 years pass through the widely used points of 25 kg/m2 and 30 kg/m2 and for adult overweight and obesity respectively [16]. In this article, we use the term “overweight” to define individuals of higher than normal weight (both obese and at-risk of obesity).
Statistical analysis
Data entry and analysis were performed using SPSS statistical software, version 11.5. Fisher exact test was used to compare prevalences between age groups and between boys and girls. Student t-test was used to compare between group means and standard deviation (SD) of continuous variables.
Results
The sample comprised 150 (50.0%) boys and 150 (50.0%) girls in the following age groups: 141 (47.0%) aged 11–14 years and 159 (53.0%) aged 15–18 years.
Obesity and at-risk of obesity
According to the BMI classification, 18.7% of students were at-risk of obesity, while 3.0% were obese. The 11–14-year-olds had a higher prevalence of at-risk of obesity than the 15–18-year-olds (23.4% versus 14.5%) but this did not reach statistical significance (P = 0.092). Compared with girls, boys had significantly higher rates of obesity (4.7% versus 1.3%) and at-risk of obesity (26.7% versus 10.7%) (P < 0.001) (Table 1).
In stratified analysis, there were no significant differences in the proportions who were overweight between the 2 age groups of boys, whereas for girls the rate of overweight (obese plus at-risk of obesity) was 17.8% in girls 11–14 years old versus 6.5% in those aged 15–18 years old (P = 0.005) (Table 1).
Table 1 Obesity status of students by age group and sex
Glycaemia, diabetes and obesity status
Out of 300 students, 31 (10.3%; 95% CI: 7.1%–14.3%) had a random capillary blood glucose level ≥ 140 mg/dL. Of 143 students reporting that they were fasting on the day of the study, 123 (86.0%, 95% CI: 80.3%–91.7%) had a fasting glucose level < 100 mg/dL (normal) 15 (10.5%, 95% CI: 5.0%–15.5%) had glucose 100–125 mg/dL (glucose intolerance) and 5 (3.5%, 95% CI: 1.1%–8.0%) had glucose ≥ 125 mg/dL (diabetes).
Figure 1 shows the association between overweight and diabetes status. There was a significant increase in the risk of prediabetes (29.6%, relative risk = 4.93) and diabetes (7.4%, relative risk = 2.85) in overweight versus normal weight students (P = 0.001).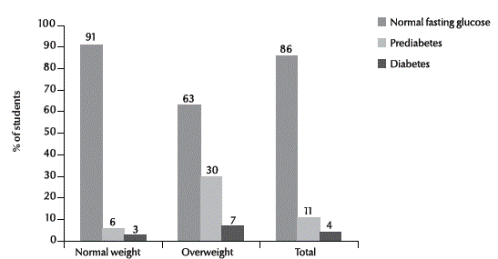
Discussion
In this study we found high rates of adolescents, particularly boys, suffering from overweight. We also found alarming rates of abnormal random and fasting glycaemia. Capillary blood glucose levels were significantly higher in students who were overweight than those who were normal weight. Three years after the 2005 study, the pattern of obesity and at-risk of obesity in Lebanese private schools and the differences between boys and girls are the same; boys were more obese than girls, and age differences in obesity were significant only in girls, with higher ages associated with lower rates of overweight [9,10]. Our results are also comparable to those of Sebai et al. in Lebanon for boys and girls: 22.5% versus 16.1% for overweight and 7.5% versus 3.2% for obesity respectively [17]. They are also comparable to those reported for the USA population, with rates of obesity of 10.9% and at-risk of obesity of 22% in children aged 6–17 years [18].
The rates of diabetes were somewhat higher than those found elsewhere in the world for children and adolescents [2–6]. Pearson et al. performed a similar study in Copenhagen schools and found similar patterns of obesity and overweight, although few students had abnormal glycaemia [19]. In addition, the SEARCH for Diabetes in Youth study group in the year 2001 estimated the overall prevalence of type 2 diabetes in youth aged 10–19 years as 0.42 per 1000 (95% CI: 0.39–0.45), with considerable variability across race/ethnic groups [20]. These figures are different from those reported by the Third National Health and Nutrition Examination Survey; their estimated prevalence of diabetes per 100 adolescents aged 12–19 years was 0.41% (95% CI: 0%–0.86%). The prevalence of impaired fasting glucose (≥ 6.1 mmol/L) among adolescents without diabetes who had fasted for at least 8 h was 1.76% (95% CI: 0.02%–3.50%) [21].
Another study in the Middle East found a prevalence of impaired glucose tolerance tests of 0.25% in individuals < 14 years and 0.21% in those 14–29 years. It also found a diabetes prevalence of 0.12% and 0.79% respectively in these age groups [22]. Our results of 10.5% for impaired glucose and 3.5% for hyperglycaemia are much higher than those reported in developed or developing countries [22,23]. This suggests that immediate interventions are needed in adolescents in Lebanese schools to diagnose and treat DM.
All the cases of diabetes were previously undiagnosed, since having diabetes was an exclusion criterion, but we could not make a specific diagnosis for diabetes, to know if it was type 1 or type 2 DM. However, since glycaemia abnormalities were more common in overweight individuals and the symptoms presented by these adolescents (such as slow wound healing, blurred vision) were not severe enough to stimulate immediate help-seeking, we have reason to believe that they had impaired glucose tolerance or hyperglycaemia caused by higher insulin resistance associated with type 2 DM [24,25]. In fact, insulin resistance is a common feature of childhood obesity and is considered to be an important link between adiposity and the associated risk of type 2 DM, metabolic syndrome and cardiovascular disease. Several factors are implicated in the pathogenesis of obesity-related insulin resistance, such as increased free fatty acids and hormones and cytokines released by adipose tissue [26].
In addition, there is a possibility of a non-differential classification bias, since the apparatus we used to measure glycaemia may be less sensitive or specific than regular laboratory measurements. While we have no reason to believe that the results would change if we use more accurate tests, there is a possibility of improvements in sensitivity [12] that could provide higher estimates of the prevalence of diabetes.
Nevertheless, further larger-scale studies are needed to confirm the findings of this pilot study and provide more evidence of the need to assess and diagnose diabetes in the Lebanese adolescent population. In the meantime, prevention of type 2 DM by healthy eating habits and physical activity education are necessary for all adolescents [13]. Screening should also be regularly performed according to worldwide recommendations, such as those of the American Diabetes Association [27].
In conclusion, we found high rates of both obesity and diabetes; therefore, diagnosis and treatment of both these problems in Lebanon should be a priority for children and adolescents and should be offered to individuals who need it.
References
- Dietz WH. Overweight in childhood and adolescence. New England Journal of Medicine, 2004, 350:855–857.
- Fagot-Campagna A. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. Journal of Pediatric Endocrinology & Metabolism, 2000, 13(Suppl, 6):1395–1402.
- Savage PJ et al. High prevalence of diabetes in young Pima Indians: evidence of phenotypic variation in a genetically isolated population. Diabetes, 1979, 28:937–942.
- Drake AJ et al. Type 2 diabetes in obese white children. Archives of Disease in Childhood, 2002, 86:207–208.
- Wabitsch M et al. Type II diabetes mellitus and impaired glucose regulation in Caucasian children and adolescents with obesity living in Germany. International Journal of Obesity, 2004, 28:307–313.
- Malecka-Tendera E, Erhardt E, Molnar D. Type 2 diabetes mellitus in European children and adolescents. Acta Paediatrica, 2005, 94:543–546.
- Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity, 2008, 15:21–29.
- Weiss R et al. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care, 2005, 28:902–909.
- Chakar H, Salameh P. Growth and obesity in Lebanese private schools children. Le Journal Medical Libanais, 2007, 55:75–82.
- Chakar H, Salameh P. Obesity in Lebanese private schools adolescents. European Journal of Public Health, 2006, 16(6):648–651.
- Chakar H, Salameh P. Growth and obesity in Lebanese public schools adolescents. Lebanese Medical Journal (Article accepted for publication).
- Rolka DB et al. Performance of recommended screening tests for undiagnosed diabetes and dysglycemia. Diabetes Care, 2001, 24:1899–1903.
- Schwartz MS, Chadha A. Type 2 diabetes mellitus in childhood: obesity and insulin resistance. Journal of the American Osteopathic Association, 2008, 108:518–524.
- Magarey AM et al. Predicting obesity in early adulthood from childhood and parental obesity. International Journal of Obesity and Related Metabolic Disorders, 2003, 27:505–513.
- Luciano A et al. Definition of obesity in childhood: criteria and limits. Minerva Pediatrica, 2003, 55:453–459.
- Cole TJ et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal, 2000, 320:1240–1243.
- Sibai AM et al. Prevalence and covariates of obesity in Lebanon: findings from the first epidemiological study. Obesity Research, 2003, 11:1353–1361.
- Styne D. Childhood and adolescent obesity. Prevalence and significance. Pediatric Clinics of North America, 2001, 48:823–854.
- Pearson S et al. Screening Copenhagen school children at risk of type 2 diabetes mellitus using random capillary blood glucose. Acta Paediatrica (Oslo, Norway), 2007, 96:885–889.
- SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics, 2006, 118:1510–1518.
- Fagot-Campagna A et al.; Third National Health and Nutrition Examination Survey. Diabetes, impaired fasting glucose, and elevated HbA1c in U.S. adolescents: the Third National Health and Nutrition Examination Survey. Diabetes Care, 2001, 24:834–837.
- El-Hazmi MA, Warsy AS. Prevalence of overweight and obesity in diabetic and non-diabetic Saudis. Eastern Mediterranean Health Journal, 2000, 6:276–282.
- Singh R, Shaw J, Zimmet P. Epidemiology of childhood type 2 diabetes in the developing world. Pediatric Diabetes, 2004, 5:154–168.
- Caprio S et al. Early phase insulin secretion is normal in obese adolescents with impaired glucose tolerance (IGT). Program and abstracts of the 61st Scientific Sessions of the American Diabetes Association; June 22-26, 2001; Philadelphia, Pennsylvania. Diabetes, 2001, 50(Suppl. 2):Abstract 224OR.
- Botero D, Wolfsdorf JI. Diabetes mellitus in children and adolescents. Archives of Medical Research, 2005, 36:281–290.
- Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. European Journal of Endocrinology, 2008, 159(Suppl. 1):S67–S74.
- American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care, 2000, 23:381–389.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)