Research article
A.A. Al-Sulami,1 A.M. R. Al-Taee 2 and M.G. Juma’a 3
استفراد وتشخيص جرثومة الـمَلْويَّة البوّابية في مياه الشرب في محافظة البصرة بالعراق
أمين عبد الجبار السُّلَمي، أسعد محمد رضا الطائي، ميساء غازي جمعة
الخلاصة: إن طرز انتقال العدوى بالملويَّة البوابية مازالت غير واضحة. وقد جُمعت 198 عينة لمياه الشرب من 22 منطقة في محافظة البصرة خلال الفتـرة من تشرين الأول/أكتوبر 2006 حتى تموز/يوليو 2007. وقِيسَ تركيز الكلور المتبقي وكذلك عدد جراثيم القولونيات الإجمالية والبرازية. وقد حصل الباحثون على 469 مزرعة جرثومية، بعد استزراع العينات على مُسْتَنْبَت كولومبيا الأغار بالدم المحوَّر، وأمكن التعرُّف فيها على 173 مستفردةً جرثومية، أربع عشرة منها كانت لأنواع الـمَلْويَّة، منها عشرة فقط للمَلْويّة البوّابية (%2.0 من إجمالي المستفرَدات). وتم اختبار هذه المستفرَدات من حيث حساسيتها للمضادات الحيوية وقدرتها على تحمل الكلور بتـركيز 0.5 مغ/ل. وهذه - حسب معلومات الباحثين – هي أول مرة يتم الإبلاغ فيها عن وجود جرثومة الملوية البوابية في مياه الشرب المعالَجة.
ABSTRACT The mode of the transmission of Helicobacter pylori infection remains poorly understood. A total of 198 samples of drinking water from 22 districts of Basra governorate were collected during the period October 2006 to July 2007. The concentration of residual chlorine was measured and the numbers of total and faecal coliforms were counted. On modified Columbia urea agar, 469 bacterial cultures were obtained, of which 173 isolates were identified. Only 14 isolates were Helicobacter spp., of which 10 were H. pylori (2.0% of the total isolates). These isolates were tested for antibiotic susceptibility as well as ability to tolerate chlorine at 0.5 mg/L. To our knowledge, this is the first report of the occurrence of H. pylori in treated municipal drinking water.
Isolation et identification d’Helicobacter pylori dans l’eau potable du gouvernorat de Bassora (Iraq)
RÉSUMÉ Le mode de transmission d’une infection à Helicobacter pylori reste mal connu. Cent quatre-vingt-dix-huit échantillons d’eau potable provenant de 22 districts du gouvernorat de Bassora ont été recueillis entre octobre 2006 et juillet 2007. La concentration de chlore résiduel a été mesurée et le nombre de coliformes totaux et fécaux ont été comptés. Sur les 469 cultures bactériennes obtenues à partir de la gélose Columbia modifiée à l’urée, 173 isolats ont été identifiés. Seuls 14 d’entre eux correspondaient à Helicobacter spp., dont 10 à H. pylori (2 % du total des isolats). La sensibilité aux antibiotiques de ces isolats a été testée, de même que leur tolérance au chlore à une concentration de 0,5 mg/l. À notre connaissance, il s’agit du premier signalement d’H. pylori dans de l’eau potable municipale traitée.
1Department of Biology, College of Education, University of Basra, Basra, Iraq (Correspondence to Al-Sulami:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
2Department of Marine Environmental Chemistry, Marine Science Centre, Basra, Iraq.
3College of Medicine, University of Missan, Missan, Iraq.
Received: 30/10/08; accepted: 16/03/09
EMHJ, 2010, 16(9):920-925
Introduction
Helicobacter pylori, originally classified as Campylobacter pylori, is a Gram-negative, microaerophilic, spiral-shaped, motile bacterium associated with gastritis, peptic ulcer, duodenal ulcer and chronic gastritis. It is also implicated in the development of gastric cancer [1–3]. The mode of transmission of H. pylori remains poorly understood. It has been suggested that the housefly has the potential to transmit the bacterium, especially in areas of the world with poor sanitation [4]. Other likely transmission routes are faecal–oral, iatrogenic or oral–oral [5]. While drinking water contaminated with faeces has been proposed as a source of infection, H. pylori has not been isolated from water except in some instances in which it was detected using polymerase chain reaction (PCR) on samples from Colombia, Lima and Peru [6,7].
Three epidemiological studies in South America have linked transmission to food and water. In Chile, more than 60% of 1815 Chileans younger than 35 years old and of lower socioeconomic groups were found to be H. pylori seropositive [8]. A study of 407 children aged 2 months to 12 years in Peru also concluded that water was the vehicle of infection, because children who used the municipal water supply had a higher prevalence of H. pylori infection than children who used private wells [9]. Furthermore, an increased risk of infection was observed in children who swam in rivers and streams in the southern Colombian Andes [10]. While all these studies confirmed the possibility of H. pylori transmission via water, efforts to isolate the bacterium from water have been unsuccessful [11,12].
This study in Basra, Iraq, aimed to isolate H. pylori from treated drinking water and investigate the relationship of H. pylori to total and faecal coliforms as well as its susceptibility to several antibiotics and to chlorine.
Methods
Enumeration and identification of H. pylori
A total of 198 samples of drinking water from 22 districts in Basra governorate were collected during the period October 2006 to July 2007.
The concentration of residual chlorine for each sample was measured using a chlorine meter (Lovibond 2000). Aliquots of 250 mL from each sample were filtered by the membrane filtration technique using 47 mm cellulose acetate filters with a nominal pore size of 0.22 µm (Sartorius). The filter papers were cultured on modified Columbia urea agar medium [13] consisting of Columbia agar supplemented with 1% haemin, 5% urea solution, 4 µg of vancomycin and 0.12 mg of phenol red and incubated at 37 ºC for 5–7 days under microaerophilic conditions (5% CO2, 10% H2, 85% N2) for the isolation of H. pylori.
H. pylori was identified using biochemical tests which included: the catalase, oxidase and urease tests, tests for hydrogen sulphide (H2S) production, nitrate reduction, growth with 3.5% NaCl, growth with 1% glycine, growth at varying temperatures (25 °C and 42 °C), growth on peptone-starch-dextrose agar and sensitivity to cephalothin and nalidixic acid.
H. pylori isolates were tested for their antibiotic susceptibility according to the method of Piddock [14] using 7 antibiotics disks including tetracycline, ampicillin, amoxicillin, erythromycin, kanamycin, gentamicin and rifampicin (Bioanalyze).
The isolates of H. pylori were exposed to 0.5 mg/L concentrations of chlorine for 4 different time periods (10s, 20s, 40s and 60s) [15].
Enumeration of coliform bacteria
The concentrations of coliform bacteria were determined by filtering 2 × 10 mL volumes of each sample using 47 mm cellulose acetate filters with a nominal pore size of 0.45 µm (Sartorius). The filters were cultured on m-FC agar and m-Endo agar for detection of faecal and total coliforms respectively.
Results
A total of 198 water samples were collected from 22 different districts during the period of the study which extended over the winter and summer seasons.
Measurement of residual chlorine concentration in the water samples showed that 41.3% of samples were free of chlorine. Figure 1 shows the residual chlorine concentrations in the districts in the winter and summer seasons. In some of the districts the concentrations varied greatly between the seasons and were usually higher in summer than winter.
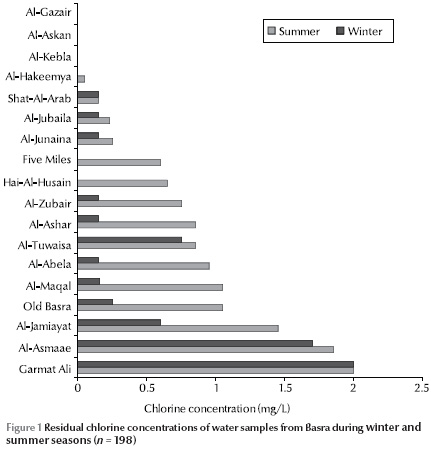
Only 14.1% of the samples conformed to World Health Organization criteria for water quality of zero fecal and total coliforms [16] (Table 1). Only 80/198 isolates were positive for the 3 biochemical tests for H. pylori positivity (urease, catalase and oxidase). On completing the other biochemical tests only 14 isolates were characterized as Helicobacter spp.: 4 H. mustelae and 10 H. pylori (Table 2). Therefore isolates of H. pylori comprised 2.0% of the total isolates and 5.0% of the total samples. The presence of these isolates was higher in certain districts than others, especially in Old Basra, Al-Jamiayat, Al-Zubair and Al-Ma’aqal.
Antibiotic susceptibility tests showed that 80% of H. pylori isolates were susceptible to tetracycline, 50% to ampicillin and amoxicillin, 40% to kanamycin, gentamicin and rifampicin and 30% to erythromycin. Inactivation of H. pylori isolates by chlorination showed that H. pylori were not sensitive to chlorine, since the final numbers of bacterial colonies were high after each period of chlorine exposure, i.e. less than 1-log reduction (Table 3).
Discussion
Culture is considered the gold standard for detection of bacteria, but the method is not sensitive, and is specific only if additional testing is performed on the isolates. However, the application of molecular biology identification methods has potential drawbacks [16]. The method of choice involves PCR amplification of specific H. pylori genes such as ureA and ureC. Although this technique appears to be sensitive, it lacks specificity [17]. Therefore other approaches have been reported involving the use of H. pylori 16s rRNA [18,19] or different sets of primers [16].
The present study was intended as a preliminary probing for the presence of H. pylori in drinking water. A more comprehensive research project on both drinking water and sewage is underway, for which a combination of biochemical and PCR using ureA primer gene and rRNA gene primer is applied.
The mode of transmission of H. pylori remains an area of discussion. Increased risk of infection has been associated with contaminated drinking water [9] and the consumption of uncooked vegetables irrigated with untreated sewage [8]. Currently the role of water in dissemination of this pathogen remains problematic since H. pylori is a fastidious organism and has been difficult to isolate from environmental sources such as water [15].
Studies of the presence of H. pylori in the aquatic environment have relied on molecular methods using PCR, immunomagnetic separation and autoradiography [20,21]. These studies suggest that the organism may survive in water for an extended period of time and that H. pylori infection is spread by contaminated water [15]. The present study succeeded in isolating H. pylori from chlorine-treated drinking water by using culture methods. We can link this to the low concentration of chlorine in the water samples, permitting it to grow and survive in large numbers, and to the water distribution system, since this bacterium has the ability to form biofilms in waterpipes [22,23]. Furthermore, the waterpipes suffer from breaks and corrosion at many sites, which cause drinking water contamination by sewage infiltration and rain leakage into the system [24,25].
The higher isolation rate of this bacterium in some districts than others may be due to the increased breaks and corrosion sites in the water supply systems which raise the rate of contamination. In some of these samples H. pylori were detected in the absence of coliforms, suggesting the shortcomings of these indicators for pathogenic bacteria.
Another explanation for the high isolation rate of H. pylori is the use of modified Columbia urea agar medium, which may enhance the growth of this bacterium.
Isolates of H. pylori were 80% tetracycline sensitive. This resistance may be a result of mutation in the 16rRNA gene, which is the target for this antibiotic [26]. For kanamycin, gentamicin, erythromycin and rifampicin the resistance could be a result of gene mutation such as mutation of the rpoB gene which is the target for rifampicin. Generally antibiotic resistance is considered the main problem associated with H. pylori treatment since antibiotic-resistant strains have become prevalent throughout the world and are the main cause of failure in H. pylori treatment.
Increased resistance of H. pylori to chlorine may be attributed to the growth of bacterial cells in the form of biofilms that make these cells acquire greater resistance to disinfectants than free cells [27]. However, some studies found that H. pylori isolates were resistant to chlorine and ozone but sensitive to monochloramine disinfection [28].
To our knowledge, this is the first report of the isolation of H. pylori in municipal treated drinking water and this could be of epidemiological significance. Further research is needed to establish which factors affect the ability of H. pylori to survive in distribution systems and be isolated from drinking water, such as the bacterial strains, density of bacteria in the distribution systems, type of waterpipe materials, efficiency of disinfection process and the techniques and materials used for culture.
References
- Goodwin CS. Campylobacter pylori, detection and culture. In: Rathbone BJ, Healthy RV, eds. Campylobacter pylori and gastro-duodenal disease. Oxford, Blackwell Scientific Publications, 1989:60–62.
- Guidelines for drinking-water quality, 3rd ed. Volume 1: Recommendations. Geneva, World Health Organization, 2004.
- Lavigne A, de Reuse H. Determination of Helicobacter pylori pathogenicity. Infectious Agents and Disease, 1996, 5:191–202.
- Grubel P et al. Vector potential of houseflies (Musca domestica) for Helicobacter pylori. Journal of Clinical Microbiology, 1997, 35:1300–1303.
- Dunn BE et al. Helicobacter pylori. Clinical Microbiology Reviews, 1997, 10:720–741.
- Hulten K et al. Helicobacter pylori in the drinking water in Peru. Gasroenterology, 1996, 110:1031–1035.
- Schauer DB et al. Detection of Helicobacter pylori in drinking water using polymerase chain reaction amplification. Gut, 1995, 37:A27.
- Hopkins RJ et al. Seroprevalence of Helicobacter pylori in Chile: vegetables may serve as one route of transmission. Journal of Infectious Diseases, 1993, 168:222–226.
- Klein PD et al. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet, 1991, 337:1503–1506.
- Goodman KJ et al. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. American Journal of Epidemiology, 1996, 144:290–299.
- Adams BL et al. Survival of Helicobacter pylori in a natural freshwater environment. Applied and Environmental Microbiology, 2003, 69:7462–7466.
- Engstrand L. Helicobacter in water and waterborne routes of transmission. Journal of Applied Microbiology, 2001, 90:80S–84S.
- Al-Sulami A et al. Primary isolation and detection of Helicobacter pylori from dyspeptic patients: a simple, rapid method. Eastern Mediterranean Health Journal, 2008, 14 (2):268–276.
- Piddock LJJ. Techniques use for the determination of antimicrobial resistance and sensitivity in bacteria. Antimicrobial Agents Research Group. Journal of Applied Microbiology, 1990, 68:307–318.
- Johnson CH et al. Inactivation of Helicobacter pylori by chlorination. Applied and Environmental Microbiology, 1997, 63:4969–4970.
- Liu H et al. Specific and sensitive detection of H. pylori by real time RT-PCR and in situ hybridization. PLoS ONE, 2008, 3(7):e2689.doi:10.1371/J.
- Camorlinga-Ponce M et al. Topographical localisation of cagA positive and cagA negative Helicobacter pylori strains in the gastric mucosa; an in situ hybridisation study; an in situ hybridization study. Journal of Clinical Pathology, 2004, 57:822–828.
- Kolbert CP, Persing DH. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Current Opinion in Microbiology, 1999, 2:299–305.
- Smith SI et al. Comparison of three PCR methods for detection of Helicobacter pylori DNA and detection of cagA gene in gastric biopsy specimens. World Journal of Gastroenterology, 2004, 10:1958–1960.
- West AP, Millar MR, Tompkins DS. Effect of physical environmental on survival of Helicobacter pylori. Journal of Clinical Pathology, 1992, 45:228–231.
- Shahamat M et al. Use of autoradiography to assess viability of Helicobacter pylori in water. Applied and Environmental Microbiology, 1993, 59:1231–1235.
- Al-Taee, M.R. Assessment of water quality due to microbial growth in drinking water distribution systems in Basrah city. Marina Mesopotamica, 2001, 16(1):37–46.
- Momba MNB, Makala N. Comparing the effect of various pipe materials on biofilm formation in chlorinated and combined chlorine-chloraminated water systems. Water SA, 2003, 30(2):175–182.
- Geldreich EE et al. Searching for a water supply connection in the Cabool, Missouri disease outbreak of Escherichia coli 0157:H7. Water Research, 1992, 26(8):1127–1137.
- Sartory PD, Holmes P. Chlorine sensitivity of environmental, distribution system and biofilm coliforms. Water Science and Technology, 1997, 35(11–12):289–292.
- Ribeiro QML et al. Detection of high-level tetracycline resistance in clinical isolates of Helicobacter pylori using PCR-RFLP. FEMS Immunology and Medical Microbiology, 2004, 40:57–61.
- Ford TE. The microbial ecology of water distribution and outfall systems. In: Ford TE, ed. Aquatic microbiology: an ecological approach. London, Blackwell Scientific, 1993:455–482.
- Baker KH, Hegarty JP. Presence of Helicobacter pylori in drinking water is associated with clinical infection. Scandinavian Journal of Infectious Diseases, 2001, 33:744–746.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)