Case report
M.M. Mukhtar,1 E.M. Elamin,2 S.M. Bakhiet,1 M.M. Kheir 3 and A.B. Ali 4
1Institute of Endemic Diseases; 3Faculty of Medicine, University of Khartoum, Khartoum, Sudan (Correspondence to M.M. Mukhtar: This e-mail address is being protected from spambots. You need JavaScript enabled to view it ).
2AlZaiem Alazhari University, Khartoum, Sudan.
4Faculty of Medicine, Al Nilian University, Khartoum, Sudan.
Received: 23/01/08; 29/06/08
Introduction
Leishmania/HIV coinfection is a new clinical form of leishmaniasis that has been reported in more than 35 countries [1,2]. The World Health Organization (WHO) estimates that 39.5 million people are infected by HIV worldwide and that one-third of them live in Leishmania-endemic regions [1,2]. Most of the reported Leishmania/HIV coinfections were among patients infected with viscerotropic parasites, but few data are available on L. major/HIV coinfection [2]. Coinfection of HIV patients with Leishmania spp. can occur naturally through infected vectors or artificially among intravenous drug users and recipients of blood transfusion. The coinfection modulates the severity of the clinical presentation of leishmaniasis and interferes with proper diagnosis. Leishmania/HIV coinfection of patients can result in the emergence of diverse Leishmania parasite clones; suppresses the host immune response; and increases blood parasitaemia, hence enhancing transmission. The coinfection also reduces the response of patients to antileishmanial drugs [3].
Cutaneous leishmaniasis is a neglected clinical form of leishmaniasis in Sudan. It is endemic in the north, central and western regions of the country. Cutaneous leishmaniasis in Sudan is thought to be caused by Leishmania major and transmitted by Phlebotomus papatasi. Recently L. donovani has been identified as a cause of cutaneous leishmaniasis in Sudan, although the vector has not yet been identified [4].
HIV is a growing health problem in Sudan with an increasing prevalence in most regions of the country. In this manuscript we report the first patient diagnosed with L. major/HIV coinfection in Sudan.
Case report
This case report emerged from a large study on biotechnological typing of leishmania parasites in Sudan. The typing study was approved by the National Ethical Committee of the Federal Ministry of Health, Sudan. The approved protocol included obtaining consent of the participants for sample collection and HIV testing for unusual and severe clinical presentations to assist effective treatment and control. The patient in this case report gave written consent for HIV testing and enrolment in the typing study. Based on the severity of the presenting lesions he was suspected of HIV coinfection and was referred to the national HIV control programme for pre-counselling and HIV testing. Following the diagnosis of Leishmania/HIV coinfection, the patient was provided with HIV counselling and free treatment by the national HIV control programme according the national guidelines.
A 38-year-old Sudanese male was referred to our laboratory at the Institute of Endemic Diseases during the last week of December 2007 for diagnosis of persistent ulcers. On clinical examination, the patient presented with 5 ulcers on his right arm, 3 on his left arm and a single lesion on his back. The ulcers were large, with necrotic and haemorrhagic zones (Figure 1). The patient had no fever or enlarged lymph nodes. The spleen and the liver were normal.
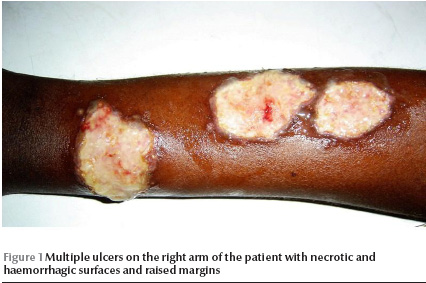
Tissue aspirates were collected from the periphery of the ulcers for preparation of smears and parasite culture. The parasite was typed based on the size of the amplified kDNA band using the method described by Smyth et al. [5].
The Leishmanin skin test was done as described by Sokal [6] but was found to be negative. The presence of anti-HIV antibodies was determined using an enzyme-linked immunosorbent assay (ELISA) and Western blotting.
Leishmania/HIV coinfection was confirmed in the second week of January 2008. The patient was referred to the national HIV control programme for counselling and treatment.
Discussion
Our patient presented with unusually large multiple ulcers on both arms and his back. The ulcers had persisted for more than 3 months and were unresponsive to antibiotics. The patient lived in an area in central Sudan endemic for cutaneous leishmaniasis, which is about 100 km south of the capital, Khartoum. He had no history of travel to other parts of the country. Cutaneous leishmaniasis was confirmed by demonstration of the parasite in the lesion aspirates, which showed high parasitaemia. Furthermore, the parasite was successfully cultured in NNN media within 48 hours of inoculation of the media. The parasite was identified as L. major based on the amplification of a kDNA band of 700 bp identical to the reference strain [5]. In Sudan, both L. major and L. donovani parasite complexes have been reported to cause cutaneous leishmaniasis in central, north and western Sudan [4]. The patient was confirmed with HIV infection based on the detection of significant anti-HIV antibodies using ELISA and Western blotting. The high parasite load and the lack of response to the Leishmanin skin test could be due to the immunodeficiency caused by HIV infection. Interestingly, no evidence of visceralization of the parasite was detected. The patient did not have a fever; he had a normal spleen and liver and no lymphadenopathy, which are the clinical signs of visceral leishmaniasis [7,8]. The finding indicates a restricted dermal-tropism of L. major isolates in Sudan.
Leishmania/HIV coinfection is a growing, serious health problem. Besides their resistance to conventional treatment, coinfected patients are a rich source for the transmission of the parasite. To our knowledge, this is the first report on L. major/HIV coinfection in Sudan and such coinfection should be considered in the management and control of cutaneous leishmaniasis in Sudan given the current surge of HIV infection.
Acknowledgement
The study received partial financial support from the joint WHO Eastern Mediterranean Region (EMRO), Division of Communicable Diseases (DCD) and the WHO Special Programme for Research and Training in Tropical Diseases (TDR): the EMRO/DCD/TDR Small Grants Scheme for Operational Research in Tropical and Communicable Diseases (grant number A20743 and grant number 990559).
References
- Report on the consultative meeting on Leishmania/HIV co-infection, Rome 6–7 September 1994. Geneva, World Health Organization, 1995:1–14.
- Alvar J et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clinical microbiological reviews, 2008, 21(2):334–59.
- Lopez-Velez R. Clinico-epidemiologic characteristics, prognostic factors, and survival analysis of patients coinfected with human immunodeficiency virus and Leishmania in an area of Madrid, Spain. American journal of tropical medicine and hygiene, 1998, 58:436–43.
- Elamin EM et al. Identification of Leishmania donovani as a cause of cutaneous leishmaniasis in Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2008, 102(1):54–7.
- Smyth AJ et al. Rapid and sensitive detection of Leishmania kinetoplast DNA from spleen and blood samples of kala azar patients. Parasitology, 1992, 105:183–92.
- Sokal JE. Measurement of delayed skin test responses. New England journal of medicine, 1975, 293:501–2.
- Russo R. Visceral leishmaniasis in those infected with HIV: clinical aspects and other opportunistic infections. Annals of tropical medicine and parasitology, 2003, 1:S99–105.
- Zijlstra E, Elhassan AM. Leishmaniasis in Sudan: visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2001, 95(Suppl. 1):S1/27–1/58.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)