Research article
K. Arkoob,1 M. Al-Nsour,2 O. Al-Nemry 3 and B. Al-Hajawi 2
وبائيات سرطان الثدي لدى النساء: خصائص المريضات وتحاليل البقاء على قيد الحياة، الأردن
كمال أبو عرقوب، مهنَّد النسور، عمر النّمري، بسام الحجَّاوي
الخلاصـة: قاس الباحثون معدل البقاء على قيد الحياة لمدة خمس سنوات لدى المصابات بسرطان الثدي في الأردن، وتعرفوا على العوامل المؤثِّرة فيه. وقد شملت الدراسة جميع الأردنيات اللاتي شُخِّص لديهن سرطان الثدي في العامين 1997 – 1998. وحصل الباحثون على المعطيات بطريقة اسْتِعادية من السجل الأردني للسرطان ومن سجلات المستشفيات. وبلغ عدد النساء اللاتي شملتهن الدراسة 838 امرأة، شُخِّص لدى نصفهنّ سرطان الثدي وهنّ في عمر 40 – 59 عاماً. وبلغ إجمالي معدل البقاء على قيد الحياة لمدة خمس سنوات وفق كابلان – ماير: 59.3%. واتضح أن لكل من المرحلة التي بلغها المرض، ووحدانية الجانب بالنسبة للإصابة، ودرجة الإصابة أثراً يُعْتَدُّ به إحصائياً على معدل البقاء على قيد الحياة، إلا أن التَّحَوُّف النسبي بحسب كوكس قد أظهر أن المرحلة التي بلغها الورم تمثِّل العامل الوحيد الذي يؤثر تأثيراً يُعْتَدُّ به إحصائياً على تحليل البقاء على قيد الحياة بعد ضبط العوامل الأخرى، وتمس الحاجة لإجراء المزيد من الدراسات للتأكُّد من النتائج، ولضمان إدراج العوامل الأخرى في الحسبان.
ABSTRACT The 5-year survival rate of female breast cancer cases in Jordan and some of the factors that affected survival were measured. All Jordanian women newly diagnosed with breast cancer during 1997–98 were included. Data were obtained retrospectively from the Jordan Cancer Registry and hospital records. Of 838 women included, half were diagnosed between the ages 40 and 59 years. The overall Kaplan–Meier 5-year survival rate was 59.3%. Stage, laterality and grade had a significant effect on survival rate. However, Cox proportional regression showed that tumour stage was the only factor that significantly influenced survival analysis after controlling for other factors. Further studies are needed to confirm the results and to ensure the inclusion of other factors.
Épidémiologie du cancer du sein chez les femmes : caractéristiques des patients et analyse du taux de survie en Jordanie
RÉSUMÉ Le taux de survie à cinq ans chez les femmes atteintes d’un cancer du sein en Jordanie et certains des facteurs ayant eu une influence sur la survie ont été mesurés. Toutes les femmes jordaniennes chez lesquelles un nouveau diagnostic de cancer du sein avait été posé en 1997-1998 ont été incluses. Les données ont été obtenues rétrospectivement dans le Registre du cancer jordanien et les dossiers hospitaliers. Pour la moitié des 838 femmes incluses, le diagnostic avait été établi entre 40 et 59 ans. Le taux de survie global à cinq ans par la méthode Kaplan-Meier était de 59,3 %. Le stade, la latéralité et le grade avaient un effet important sur le taux de survie. Toutefois, le modèle de régression à effet proportionnel de Cox a révélé que le stade de la tumeur était l’unique facteur ayant une véritable influence sur l’analyse de la survie après l’élimination d’autres facteurs. Des études supplémentaires sont nécessaires pour confirmer les résultats et garantir l’inclusion d’autres facteurs.
1Al-Basheer Hospital, Ministry of Health, Jordan.
2Disease Control and Prevention Directorate, Ministry of Health, Jordan (Correspondence to M. Al-Nsour: This e-mail address is being protected from spambots. You need JavaScript enabled to view it )
3Jordan Cancer Registry, Ministry of Health, Jordan.
Received: 08/12/07; accepted: 06/01/09
EMHJ, 2010, 16(10):1032-1038
Introduction
Breast cancer is considered to be the most common cancer throughout the world. Statistics show that about 700 000 cases are reported annually, about 57% of them in developing countries [1]. Data from the United States of America indicate that the incidence of breast cancer increases directly with age until 75–80 years [2]. In Jordan, cancer is the second most frequent cause of death after cardiovascular disease [3]. According to official Jordanian statistics, breast cancer was the most common of all cancers in women in the last decade [3], a figure that agrees with the results obtained from different countries in the region [4,5].
The survival rate is one of the most important measures of cancer care, and is a valuable tool for comparisons between countries. Studies of cancer patient survival, using data from population-based cancer registries, are essential for monitoring and evaluating the effectiveness of the diagnosis and treatment of cancer [6]. The higher survival rates being recorded in some developed countries reflect improvements that have taken place in various areas of cancer control, from health education and early diagnosis to treatment and after care [7]. Information about survival rates in Jordan will help us take further preventive and control measures in order to improve the prognosis of patients with different types of cancers. The present study aim to identify the epidemiology of female breast cancer in Jordan in 1997–98; to measure the observed 5-year survival of female breast cancer cases diagnosed in Jordan in the years 1997–98; and to explore some of the factors that affect survival rates, such as age at diagnosis, type, grade and stage of tumour. The majority of studies in Jordan to date have been descriptive, and to the best of our knowledge this is the first study of survival rates at population-based registry level in Jordan.
Methods
Study design
This was a descriptive study using data from the Jordan Cancer Registry (JCR). Established in 1996, the JCR is a population-based registry that provides statistics on the incidence of cancer throughout Jordan. JCR obtains its information about patients from governmental and nongovernmental health care facilities and military hospitals throughout the country and from laboratories and the Jordan National Civil Department. Liaison personnel in the main hospitals are responsible for registering and reporting all cases of cancer by completing a special form containing demographic and medical data, which is sent to JCR.
Data collection for the current study lasted more than 3 months. All cases of breast cancer in women that were registered in the JCR and were newly diagnosed during the period 1 January 1997 to 31 December 1998 were included in the study. Cases that could not be verified by a histopathology report were excluded.
Data collection
Special data collection forms were designed for the study to collect sociodemographic information about the patient (national ID number, name, age at diagnosis, address) and information related to current cancer status (histopathology report, laterality, stage of cancer, grade and stage of the tumour, date of diagnosis, date of last visit, and outcome). The validity and reliability of the study instrument were tested in a pilot study and some variables in the data collection instrument were modified.
Patients were first identified from the JCR registry, after which the medical records and histopathology reports from the reporting hospital were consulted to complete the data. The outcome (alive or dead) was ascertained from the Civil Registration Department using the patient’s unique national ID number. Each patient was followed up until dead or alive at the end of data collection period, i.e. for up to 5 years from the date of diagnosis to the cutoff point at 31 December 2003. The follow-up endpoint was death from any cause. Some patients were lost to follow-up as no information was available about them. The final number of patients was 838. Duplication of data was avoided by using the patient’s unique national ID number and full (4) names (patient, father, grandfather and family name).
Official approval for the study was obtained from the medical ethics committee of the Ministry of Health before starting data collection. All information about patients was handled under conditions of strict confidentiality and data were analysed anonymously by ID number. No contact was made with patients or their relatives.
Definitions
Laterality was subdivided into right, left, bilateral and unknown (undetermined or data missing). Histopathology type was categorized into: ductal carcinoma, lobular carcinoma, adenocarcinoma, medullary carcinoma and unknown (undetermined type or data missing). Tumour stage was classified into 4 stages using the American Joint Committee on Cancer TNM [tumour, node, metastasis] criteria: stage I, stage II, stage III, stage VI or unknown (undetermined stage or data missing). Tumour grade was divided into 5 categories: grade 1 (well-differentiated); grade 2 (moderately differentiated); grade 3 (poorly differentiated); grade 4 (undifferentiated) and unknown (undetermined grade or data missing).
Data management and statistical analysis
Data were entered and analysed using SPSS, version 12. Frequencies of sociodemographic and clinical characteristics of all patients were obtained. The survival rates at 1-year intervals were estimated using the actuarial (life-table) method and overall survival rates using exact survival times were estimated using the Kaplan–Meier product limit technique. Log-rank testing was used to test for an association between the variables and the survival rate. P-value < 0.05 was considered statistically significant. Survival probabilities by age, type of cancer, laterality, grade, stage and treatment were obtained. Cox proportional hazard regression analysis was performed to assess the independent effect of different prognostic factors after simultaneously controlling for the effect of potential confounders. Initially all of the following variables were entered in the model: age, type, laterality, grade, stage and treatment modality.
Results
A total of 838 women with breast cancer were included in this study. The mean age of the patients was 50.2 (standard deviation 12.4) years. Slightly more than half the patients diagnosed fell into the 40–49 and 50–59 years age groups. The left breast was the most commonly affected site (44.6%). More than three-quarters (77.5%) of patients were diagnosed with ductal carcinoma and 41.7% of tumours were moderately well-differentiated. Almost half (47%) the patients received combined treatment of surgery, chemotherapy and radiotherapy. More than one-third of patients (37.6%) were in stage II at diagnosis.
The period of follow-up ranged from 1 day to 5 years. Just over half the patients (51.4%) were known to be still alive 5 years after first diagnosis, while 34.8% were recorded as dead and data were missing for 13.9% (Table 1).
Calculation of the survival rates at 1-year intervals are presented in Tables 2 and 3. Using the actuarial method, the 5-year survival rate for breast cancer cases was 59.3% (Table 2). The percentage of women surviving 1 year after diagnosis was 91.6%, for 2 years 80.1%, for 3 years 70.2% and for 4 years 65.8%.
The cumulative 5-year survival rates by the Kaplan–Meier method as a function of the different prognostic factors studied (age, type, laterality, grade, stage and treatment) are presented in Table 3. Figure 1 shows the survival curves for the same variables. The highest 5-year survival rate by age was in the age group 40–49 years (67.2%), and the poorest survival rate in the age group > 30 years (43.8%). The survival rate for patients affected in the right breast was 61.1% and for the left breast was 59.1%; however the survival rate was only 16.7% for bilateral breast cancer, a difference that was statistically significant (P < 0.001). According to the differentiation grade of the tumour, 5-year survival for well-differentiated tumours was 73.9% compared with 42.9% for undifferentiated tumours (P < 0.001). The 5-year survival rates were 71.4%, 39.6% and 5.8% for tumours in stage II, III and IV at diagnosis respectively (P < 0.001). Regarding treatment given to the patients, 5-year survival for patients receiving only surgery was slightly higher (58.3%) than for those receiving triple therapy of surgery, chemotherapy and radiotherapy (56.3%), but the difference was not significant (P = 0.45).
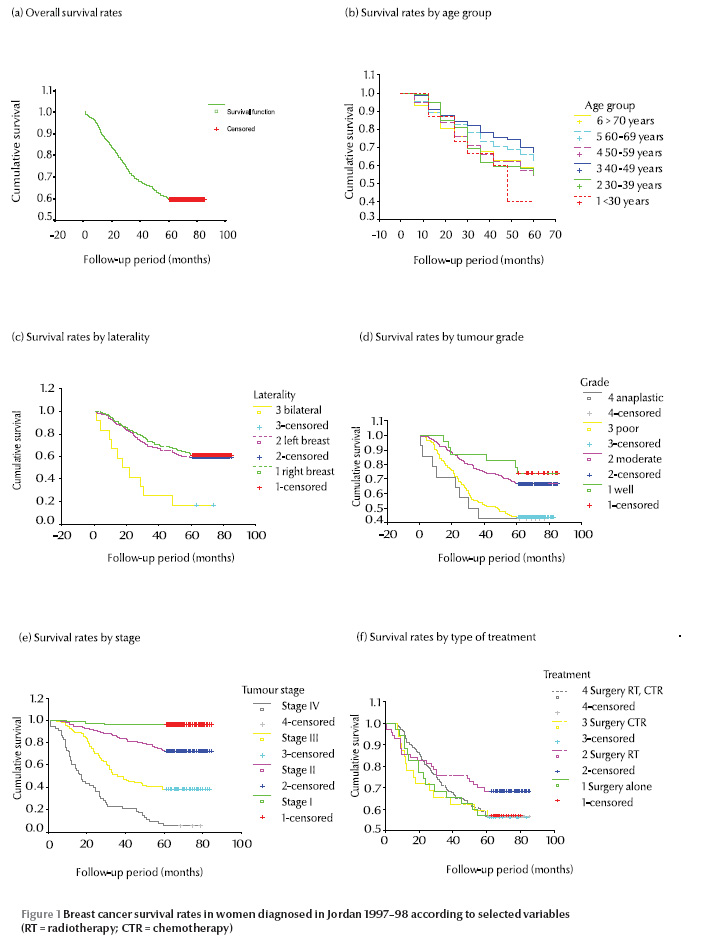
Cox proportional hazard regression analysis was performed to assess the independent effect of different prognostic factors after simultaneously controlling for the effect of potential confounders. All of the following variables were initially entered into the model—age, type, laterality, grade, stage and treatment modality—but only laterality, grade and stage of cancer were kept in the final model. Table 4 shows the hazard ratio and the significance for these 3 variables. According to tumour stage the best survival was found for stage I (hazard ratio of dying = 1.00) compared with the other stages (P < 0.001). The grade of the tumour and the laterality did not reach the level of significance in the model.
Discussion
Our study provides substantial information about the survival rate of women diagnosed with breast cancer in Jordan in 1997–98. While TNM staging at diagnosis, laterality and grade had a significant effect on survival rate, Cox proportional regression analysis showed that after controlling for other factors tumour stage was the only factor that influenced survival. Age and grade had no statistically significant association with survival.
Consistent with studies from other countries in the region [8–11], our study showed that the overall 5-year survival rate for breast cancer in Jordan, regardless of stage or grade, was 59.6%. The 1-year rate was 91.6%, the 2-year rate was 80.1%, the 3-year rate was 70.2% and the 4-year rate was 65.8%.
In line with other studies, we found that the overall survival rate was poorer in the age group 70 years (56.5%) [12–14]. Lack of competing causes of death, higher frequency of undifferentiated tumours and more cases diagnosed at stage II or III have previously been suggested as the reason for the poor survival experience of young women with breast cancer [2,15]. In contrast, a study in the United States of America about breast cancer in young patients aged < 35 years showed that overall 5- and 10-year survival rates were 65% and 49% respectively and concluded that young age was not directly related to the “aggressiveness” of breast cancer [16].
Consistent with a study from Europe [17], the best survival rate in our study was seen in the age group 40–49 years. This could be explained due to access to screening mammography in this age group or due to lower levels of circulating sex hormones, resulting in reduced stimulation of tumour cell growth [18,19].
There was a statistically significant association between survival and laterality, which was 61.1% in the right breast and 59.1% in the left breast, while in bilateral cases the 5-year survival was only 16.7%. This is probably explained by the more aggressive disease present in bilateral cases of breast cancer.
The TNM stage proved to be the most significant independent prognostic factor for determining survival. In our study only 55.6% of cases were diagnosed at stage I and II, whereas in Canada 75% were diagnosed at stage I and II [11]. Regular mammography combined with regular clinical examination may offer the best opportunity of increasing the proportion of early stage cases detected.
In our study, the 5-year survival rate varied according to stage. This was probably due to the fact that stage reflects the interaction between the host and the tumour. The frequency distribution of stage and 5-year survival by stage was similar to data reported by the American Cancer Society [18,20]. However, information from Bahrain showed that that the 5-year survival rate ranged from 87.5% at stage I to 48% at stage IV [8], which differs from the results of the present study.
A strength of our study is that it was population-based, comprising virtually all histologically verified cases registered in the JCR, and was therefore not subject to the selection bias of studies that select samples. Another important strength of the study was the ability to investigate the impact of demographic, histological and therapeutic factors on survival in breast cancer. Furthermore, the size of the study was sufficiently large to perform survival analysis across different subgroups of breast cancer cases.
On other hand, some weaknesses of our study should be addressed. First, as in other population-based studies, the medical records were not complete and there was some information missing. Although notification of cases to the JCR is mandatory, the exact cause of death was not recorded in all cases, so we assumed that cancer was the cause of death unless otherwise specified. The exact cause of death in population-based studies may not always be clearly determined.
Since 30.5% of the breast cancer cases were diagnosed at stage III and stage IV, great efforts should be made to improve this percentage through a good surveillance system and screening programmes for high-risk groups. Determining the prognosis in cancer cases depends on accurate data on prognostic factors and the appropriate choice of therapeutic and supportive interventions. Further studies are needed to confirm our results in a larger sample and perhaps to ensure the inclusion of other factors such as number of lymph nodes affected, pregnancy history, age at menarche, use of oral contraceptives, menopausal status and oestrogen and progesterone levels.
References
- World health report 1988. Life in the 21st century: a vision for all. Geneva, World Health Organization, 1998.
- Breast cancer incidence and mortality—United States, 1992. Morbidity and Mortality Weekly Report, 1996, 45(39):833–837.
- Directorate of Information Studies and REsearch. Mortality in Jordan, 2005. Amman, Ministry of Health, 2008..
- El Saghir NS et al. Age distribution of breast cancer in Lebanon: increased percentages and age adjusted incidence rates of younger-aged groups at presentation. Le Journal Medical Libanais, 2002, 50(1–2):3–9.
- Omar S et al. Breast cancer in Egypt. Eastern Mediterranean Health Journal, 2003, 9(3):448–463.
- Dickman PW, Hakulinen T. Population-based cancer survival analysis (statistics in practice series). New Jersey, John Wiley, 2000.
- Dickman PW et al. Survival of cancer patients in Finland 1955–1994. Acta Oncologica, 1998:22–25.
- Fakhro AE et al. Breast cancer: patient characteristics and survival analysis at Salmaniya Medical Complex, Bahrain. Eastern Mediterranean Health Journal, 1999, 5(3):430–439.
- Al-Moundhri M et al. The outcome of treatment of breast cancer in a developing country—Oman. Breast, 2004, 13(2):139–145.
- Vahdaninia M, Montazeri A. Breast cancer in Iran: survival analysis. Asian Pacific Journal of Cancer Prevention, 2004, 5(2):223–225.
- Ravichandran K, Hamdan NA, Dyab AR. Population based survival of female breast cancer cases in Riyadh Region, Saudi Arabia. Asian Pacific Journal of Cancer Prevention, 2005, 6(1):72–76.
- Ugant AM et al. Survival of women with breast cancer in Ottawa, Canada: variation with age, stage, histology, grade and treatment. British Journal of Cancer, 2004, 90, 1138–1143.
- Berrino F et al., eds. Survival of cancer patients in Europe: the EUROCARE study. Lyon, International Agency for Research on Cancer, 1995 (IARC Scientific Publications No.132).
- Sant M et al. Survival of women with breast cancer in Europe: variation with age, year of diagnosis and country. The EUROCARE Working Group. International Journal of Cancer, 1998, 77:679–683.
- Bonnier P et al. Age as prognostic factor in breast cancer: relationship to pathologic and biologic features. International Journal of Cancer, 1995, 62(2):138–144.
- Sariego J et al. Breast cancer in young patients. American Journal of Surgery, 1995, 170(3):243–245.
- Celko AM. Breast cancer epidemiology in the Czech Republic. Central European Journal of Public Health, 1996, 4(2):106–109.
- Coebergh JWW et al. Survival of adult cancer patients in Europe diagnosed from 1978–1989: the EUROCARE II study. European Journal of Cancer, 1998, 34:2137–2278.
- Gizlice Z. Breast cancer incidence, mortality and survival in North Carolina. SCHS Studies, 1997, September (No. 108).
- Breast cancer facts and figures 1996/1999–2000. American Cancer Society [website] (www.cancer.org/statistics/96bcffsurv.html, accessed 3 April 2010).




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)