H.I. Al-Daghistani1 and K.M. Fram2
معدَّل الأضداد المضادة للمنطقة الشفافة والمضادة للنطاف لدى الأردنيات العقيمات وعلاقته بالمفطورات
هالة إبراهيم الداغستاني، كميل موسى فرام
الخلاصـة: قيَّم الباحثون الأضداد الذاتية المضادة للمنطقة الشفافة والأضداد الإسوية المضادة للنطاف في مفرزات عنق الرحم لدى 73 من الأردنيات العقيمات و41 من النساء الشاهدات، واستخدموا لذلك التـراص باللاتكس. وقد وجدوا أن النسبة المئوية للنساء العقيمات اللاتي لديهن أضداد ذاتية مضادة للمنطقة الشفافة %16.11، وللنساء العقيمات اللاتي لديهن أضداد إسوية مضادة للنطاف %8.2، وهي نسب أعلى بكثير بالمقارنة بالنساء المخصبات اللاتي كانت نسبة من لديهن أضداد ذاتية مضادة للمنطقة الشفافة %9.4، وللنساء المخصبات اللاتي لديهن أضداد إسوية مضادة للنطاف %0، دون أن يكون هناك ارتباط بسبب العقم. وقد كشف الباحثون عن وجود المفطورة البشرية لدى %19.2 من مفرزات عنق الرحم لدى النساء العقيمات، والميوِّرة الحالة لليوريا لدى %13.7 منهن، وكان ترابط وجود المفطورة مع وجود الأضداد الذاتية المضادة للمنطقة الشفافة والأضداد الإسوية المضادة للنطاف في مفرزات عنق الرحم يعتد به إحصائياً. إن المفطورات قد تؤدِّي دوراً باعتبارها مثيراً غير نوعي للمفاويات البائية ولاسيما في وجود الأضداد الذاتية المضادة للمنطقة الشفافة.
ABSTRACT Anti-zona-pellucida autoantibodies (AZP-Ab) and anti-sperm isoantibodies (ASA) were assessed in the cervical secretions from 73 infertile Jordanian women and 41 fertile control women using latex agglutination. Significantly more women with infertility had AZP-Ab and ASA (16.4% and 8.2% respectively) compared with fertile women (9.4% and 0%), with no relation to the etiology of infertility. Using polymerase chain reaction Mycoplasma hominis and Ureaplasma urealyticum were detected in cervical secretions of 19.2% and 13.7% of infertile women, and the presence of mycoplasma was significantly correlated with the presence of AZP-Ab and ASA.
L’incidence des anticorps anti-zone pellucide et anti-sperme chez des femmes jordaniennes stériles et sa relation avec les mycoplasmes
RÉSUMÉ Les auto-anticorps anti-zone pellucide (Ac-AZP) et les isoanticorps antispermatozoïdes (AAS) ont été évalués dans les sécrétions cervicales de 73 femmes jordaniennes stériles et de 41 femmes témoins fécondes grâce à un test d’agglutination au latex. Les femmes stériles présentant des Ac- AZP et des AAS étaient significativement plus nombreuses (respectivement 16,4 % et 8,2 %) que les femmes fécondes (9,4 % et 0 %), sans qu’il y ait de relation avec l’étiologie de la stérilité. En effectuant un test PCR (amplification en chaîne par polymérase), on a détecté des bactéries Mycoplasma hominis et Ureaplasma urealyticum dans les sécrétions cervicales de 19,2 % et de 13,7 % des femmes stériles ; la présence de mycoplasmes était significativement corrélée à celle d’Ac-AZP et d’AAS.
1Department of Medical Allied Sciences, Zarka University College, Al-Balqa Applied University, Zarka, Jordan (Correspondence to H.I. Al-Daghistani:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
2Department of Obstetrics and Gynaecology, Jordan University Hospital, Amman, Jordan.
Received: 09/12/06; accepted: 03/05/07
EMHJ, 2009, 15(5):1263-1271
Introduction
The zona pellucida (ZP) is a glycoprotein membrane surrounding the oocyte and is required to activate the acrosome reaction [1]. It seems to be an important ovarian antigen that participates in the etiology of some infertility disorders, including polycystic ovarian failure [2]. Since it is formed at an early stage of oocyte growth, zona pellucida-specific autoantibody may impair ovarian function [3]. Anti-zona-pellucida autoantibodies (AZP-Ab) have been investigated among infertility cases, but their primary role remains uncertain since these antibodies might coexist along with other infertility problems [4].
The primary role of AZP-Ab remains controversial. Increased titres were found in most but not all women with unsuccessful pregnancy and were associated with ovarian dysfunction [5]. However, no correlation has been found between these antibodies and the number of oocytes and pregnancy rate [6]. Nevertheless, some studies have shown a high percentage of anti-gamete antibodies in unexplained infertility cases, and other fertility disorders [7]. Anti-sperm antibody (ASA) may also play a different role in female infertility by interfering with sperm migration through the female genital tract [8] and disrupting various stages of fertilization [9]. However, both iso- and autoantibodies might be present together in certain cases of infertility such as endometriosis [10].
Genital infection is one of the factors affecting fertility. The mycoplasmas Ureaplasma urealyticum and Mycoplasma hominis are common inhabitants of the female lower genital tract, with varying incidence in different populations [11]. Both species are linked to a wide range of diseases of the female genital tract, including infertility, [12] but their actual role in infertility has not been conclusively demonstrated.
The real significance of antibodies directed to ZP antigens remains to be established. We aimed to assess the presence of AZP-Ab and its association with ASA in infertile women with different etiologies for their infertility. In addition, we evaluated the presence of different genital pathogens, in particular M. hominis and U. urealyticum, in women harbouring ASA and AZP-Ab in their cervical secretions.
Methods
Sample
In a prospective study, cervical mucus samples were collected from 114 Jordanian females attending a private clinic in Amman during the period March 2005 to April 2006. Their median age was 25 years. The women were categorised into 2 groups: 73 infertile women presenting for an infertility evaluation, with a mean duration of infertility of 5.5 years; 41 healthy female volunteers (controls) attending the clinic for routine check-ups. Medication with antibiotics or any medication with potentially negative effects on the rheological characteristics of the mucus were stopped in the previous cycle. Women with clinical symptoms of lower genital tract infection were excluded from the study.
Informed consent was obtained from the control women.
Data collection
Cervical secretion collection
Samples of cervical secretions were collected from spontaneously ovulating infertile and fertile women. A sterile speculum was inserted into the vagina and a 5–10 mL syringe used to collect cervical secretions from the endocervical canal. Samples were placed in sterile Appendorff tubes and stored at –21 ºC until used.
Endocervical sample culture
The endocervical samples were placed in Amies transport medium to be cultivated on different culture media. Gonococci were isolated on a modified selective Thayer–Martin agar (BioMerieux, France) and a non-selective New York City agar (Oxiod, Unipath, Hampshire, England). Sugar utilization tests were carried out on all oxidase-negative diplococci. Yeast cultivation was performed using Sabouraud–Dextrose agar (Oxiod, Unipath, Hampshire, England) and identification was assessed by microscopy and the germ tube test. Staphylococcus and Streptococcus spp. were cultivated on blood agar base and full biochemical tests were used for identification. Enterobacteriaceae were identified by the API system (BioMerieux, France). All media were prepared, inoculated and incubated as routine microbiological procedures.
Assay procedure for cervical mucus AZP-Ab and ASA
AZP-Ab and ASA were measured using the latex agglutination test. The titres of AZP-Ab ranged from 1:200 to 1:1600 and for ASA from 1:400 to 1:800.
For AZP-Ab, cervical specimens were diluted 1:50, mixed and centrifuged for 10 min at 1000 g. A serial dilution of supernatant using log2 was prepared; 10 µL of antigen suspension (Bioserv, Germany) was dispensed into the marked circles on the slide and mixed with 20 µL of diluted specimen. Agglutination was recorded after 2 min.
For ASA, cervical specimens were mixed with dilution buffer (1:50), mixed and centrifuged at 1000 g for 10 min. A serial dilution of supernatant using log2 was made (1:100, 1:200 and 1:400); diluted specimen (20 μL) was added to 10 μL of antigen suspension (Bioserv, Germany) on a slide and mixed for 2 min. Agglutination was considered to be positive when sperm antibodies were present only in the specimen dilutions of 1:100 and higher.
Polymerase chain reaction
Cervical secretion samples were transferred to 0.5 mL sterile saline, and subjected to microcentrifugation at 10 000 rpm for 10 min at room temperature. Sample pellets were digested with 200 μg/mL proteinase K
in the presence of Brij detergent [13]. Samples were centrifuged and overlaid with mineral oil and incubated in a thermal cycler for 60 min at 56 °C to lyse the cells then at 95 °C for 10 min to inactivate protienase K.
Processed samples were stored at –21 °C. For M. hominis and U. urealyticum we used a published protocol incorporating oligonucleotide primer pairs specific for a 324 base-pair region of 16S ribosomal RNA gene and 224 base-pair region of the urease gene respectively [14,15].
Aliquots (25 µL) were microcentrifuged, overlaid with mineral oil, heated at 94 °C for 10 min and then immediately plugged into ice to prevent reannealing of DNA. An equal volume of reaction mixture was added to the samples and loaded in a minicycler to be subjected to PCR. Purified M. hominis and U. urealyticum were always processed and assayed in parallel to the test samples as positive controls. H2O blanks served as negative controls. Products were visualized under ultraviolet illumination on polyacrylamide gel stained with ethidium bromide [14].
Statistical analysis
Data analysis was performed with SPSS software, version 13.0. Statistical assessment of results was performed using the t-test for equality of means, Levene test for equality of variances and chi-squared test. P < 0.05 was considered statistically significant.
Results
A total of 114 women were enrolled in the study: 73 infertile and 41 healthy fertile controls. Of the infertile women, 16 (22%) were diagnosed with polycystic ovarian failure (PCO), 50 (68%) with unexplained infertility and 7 (10%) with other infertility problems (abortion for unknown reasons, hormonal imbalance, endometriosis and other causes).
AZA-Ab was detected in cervical secretions from 12 (16.4%) of the infertile women and 2 (4.9%) of the fertile women (Table 1). ASA was present in 6 (8.2%) infertile women and 0 (0.0%) fertile women and was significantly associated with infertility (P < 0.05). Both types of antibodies were detected in 2 infertile women. The prevalence of AZP-Ab was higher in females with infertility compared with fertile ones (P = 0.06), without any association with the etiology of infertility.
AZP-Ab and ASA were detected in 8 (16.0%) and 3 (6.0%) cases respectively of unexplained infertility; in 2 (12.5%) and 1 (6.3%) cases with PCO; and in 2 (28.6%) and 2 cases (28.6%) with other infertility problems. These results were not statistically significant for any single category of infertility.
Extensive microbial screening in cervical secretions from infertile women showed high percentages of Staph. aureus, Str. pyogenes, Str. faecalis, Str. viridans, Proteus mirabilis, Escherichia coli, Enterococcus spp., Pseudomonas aeruginosa, Neisseria gonorrhoeae, Candida albicans and certain commensal aerobic species (Table 2). The presence of C. albicans was significantly correlated with the presence of Streptococcus spp., P. aeruginosa and N. gonorrhoeae (P = 0.01, 0.002 and 0.031 respectively).
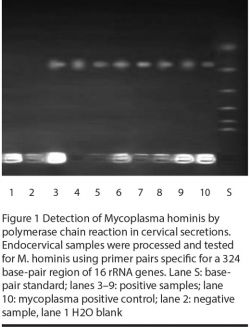
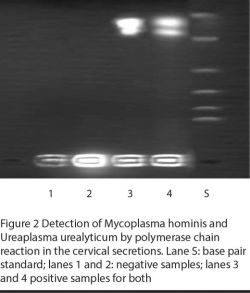
Figures 1 and 2 illustrate the PCR detection of M. hominis and U. urealyticum. M. hominis was detected (by PCR) in 14 (19.2%) infertile women and 3 (7.3%) controls, whereas U. urealyticum was detected in 10 (13.7%) infertile women and 4 (9.8%) controls (Table 3). The prevalence of both organisms was significantly related to infertility (P = 0.06 and < 0.05 respectively). Two of the infertile women harboured both mycoplasmas. In addition, the presence of mycoplasmas was significantly correlated with the presence of AZP-Ab and ASA (Table 3) and was associated more frequently with candidiasis (P = 0.001).
Discussion
Some studies have emphasized the role of gamete-specific antibodies as a possible immunopathological mechanism for reproduction failure. Antibodies to oocytes are introduced as a possible marker for this failure [16]. ZP, which is formed at the early stage of oocyte growth, seems to be one of the targets for the production of autoantibodies which might impair ovarian function [11]. It acts as an antigen and is species-specific, but with some exceptions [17,18]. Normally it does not stimulate the production of antibodies, owing to the blood–ovary barrier. Autoantibodies might be produced after the degradation of the ova, subsequently triggering an immune reaction [19].
This prospective study was designed to assess the presence of AZP-Ab in relation to PCO and unexplained infertility cases. Detectable levels of AZP-Ab and ASA were observed among infertile women (16.4% and 8.2% respectively) compared with fertile women (4.9% and 0.0% respectively). This was highly significant for ASA and infertility cases (P < 0.05), but for AZP-Ab the relation was not significant (P = 0.06). In spite of many studies emphasizing the importance of autoantibodies directed to ZP in the etiology of infertility [20], especially unexplained cases [7], we could not find any significant association between AZP-Ab and different infertility groups. However, other studies have shown a minor role for these autoantibodies, since they are only recorded in 2.4% of infertile women [4]. In patients attending an in vitro fertility programme, the presence of AZP-Ab correlated with a lower fertilization rate [21]. In most of the cases studied, however, antibodies to ZP were not correlated with those directed to sperm antigens. This might be explained by the differences in immunostimulation in production of the 2 types of antibodies. Iborra et al. showed that anti-endometrial antibodies do not cross-react with gamete antigens (sperm and oocytes) [10]. However, besides their inhibitory effect on sperm motility, some antibodies directed to sperm antigen showed inhibitory effects on ZP itself [22].
Stimulation of auto- and isoantibody formation seems to be associated with microbial infection. An increased rate of genital infection due to microorganisms has been recorded in the past few decades. Improved laboratory techniques, particularly molecular ones, have led to an increase in the recording rate of the total microorganisms isolated, especially Mycoplasma spp. However, M. hominis and U. urealyticum can be isolated with considerable frequency from the human urogenital tract and are thought to cause various problems such as nongonococcal urethritis, pelvic inflammatory disease, pyelonephritis or infertility [23]. In our study, the incidence of M. hominis as detected by PCR was 19.2% and 7.3% among infertile females and controls respectively, whereas U. urealyticum was detected in 13.7% and 9.8% respectively. The rate of detection of U. urealyticum was lower in comparison with other studies, which might be related to different populations. However, both organisms were clearly more frequent in infertile women than controls (P < 0.05). Some studies showed that vaginal colonization with U. urealyticum is much higher than with M. hominis [24]. Kapur et al. reported the recovery of U. urealyticum in 21.4% of patients suffering from vaginal discharge [25]. This variation might be explained by the differences in the pathogenicity of Ureaplasma spp. since some strains are more pathogenic than others and thus responsible for some invasive vaginal infections [26] or because the conditions in the female genitourinary tract favour the proliferation of Ureaplasma spp. [27].
While the association of fertility with genital tract colonization by Mycoplasma or Ureaplasma spp. has been extensively studied, it remains controversial. Genital tract infection is considered a non-specific immune activator leading to the immune response. This concept might be applied to our results, since M. hominis was detected in 66.6% of cases with AZP-Ab associated-infertility and only in 9.8% of the cases with no autoantibody. Also, 33.3% of infertility-related AZP-Ab showed the presence of U. urealyticum in comparison with 9.8% in those without. These facts suggest a role of mycoplasmas as nonspecific stimulators for B-lymphocytes in cervical secretions. It is well known that M. hominis has an affinity for urogenital tissue, adheres to the surface of eggs and can penetrate the ZP. This might stimulate an immune response which is followed by AZP-Ab formation [28]. Cross-reactive antigens may be present in sperm, M. hominis, testis, ovary and leukocytes [29]. In addition, Ureaplasma infection has been shown to produce a series of chromosomal changes in human lymphocytes. Both acute infection, with residual tissue damage, and reversible alterations in lymphocytes resulting from chronic colonization have been suggested as mechanisms of Ureaplasma-induced infertility [30].
Other microorganisms were investigated in the study and were shown to be present more frequently among infertile women. A significant relation was found between M. hominis and C. albicans. In addition, the presence of C. albicans was significantly correlated with Streptococcus spp., P. aeruginosa and N. gonorrhoeae. Of the microorganisms investigated, only C. albicans seemed to significantly correlate with autoantibody presence in infertile women. Repeated exposure to C. albicans in association with other types of infection (e.g. mycoplasma) may be one of the stimulators that leads to the formation of autoantibodies as a result of some antigenic cross-reactivity. The data suggest that colonization with M. hominis is likely to depend on other genital microflora, which help in creating certain environmental conditions in the cervix as well as an increased pH. This can be demonstrated even more clearly with the results obtained from examinations of infertile females harbouring C. albicans. However, this does not agree with others who reported less frequent occurrence of mycoplasmas together with genital candidiasis [24].
In conclusion, women with infertility related to autoantibodies directed towards reproductive tissue showed a significantly higher prevalence of M. hominis and U. urealyticum compared with women without autoantibody-related infertility and compared with fertile women. The association between mycoplasma infection and infertility complications leads us to speculate whether the detection of these organisms by PCR in the endocervix of women with infertility reflects its presence also in the uterus or other parts of the genital system. It is important for early diagnosis and treatment as a goal to decrease the probability of tubal occlusion and infertility. The combination of immunological and bacteriological investigations for female infertility provides important diagnostic and prognostic information and is crucial in selection of cases for in vitro fertilization and intracytoplasmic sperm injection.
Acknowledgements
We are extremely grateful to Al-Balqa Applied University for the grant sanctioned for this project. Our thanks are also due to Fatima Sousarbi, microbiology laboratory technician, for her help.
References
- Morales P, Lianos M. Interaction of human spermatozoa with the zona pellucida of oocytes: development of the acrosome reaction. Frontiers in bioscience, 1996, 1:d146–60.
- Kelkar RL et al. Circulating autoantibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. Journal of reproductive immunology, 2005, 66(1):53–67.
- Kamada M et al. Etiological implication of autoantibodies to zona pellucida in human female infertility. American journal of reproductive immunology, 1992, 28:104–9.
- Mori T et al. Possible presence of autoantibodies to zona pellucida in infertile women. Experientia, 1978, 34:797–9.
- Koyama K et al. Follicular dysfunction induced by autoimmunity to zona pellucida. Reproductive biology, 2005, 5(3):269–78.
- Cariño C et al. Localization of species conserved zona pellucida antigens in mammalian ovaries. Reproductive biomedicine online, 2002, 4(2):116–26.
- Ulcová-Gallová Z et al. Antizonalni protilatky v ovulacnim hlenu a v seru pacientek s poruchami reprodukce [Antizonal antibodies in ovulatory cervical mucus and in serum of patients with fertility disorders]. Ceská gynekologie, 2004, 69(3):215–8.
- Tsukui S et al. Blocking effect of sperm immobilizing antibodies on sperm penetration of human zona pellucida. Journal of in vitro fertilization and embryo transfer, 1988, 5:123–8.
- Arefi S et al. Anti-zona pellucida antibodies in infertile patients in relation to multiple puncture of ovaries and unexplained infertility. Iranian journal of reproductive medicine, 2005, 3(1):30–5.
- Iborra A et al. Autoimmune response in women with endometriosis. American journal of reproductive immunology, 2000, 44(4):236–41.
- McCormack WM et al. Vaginal colonization with Mycoplasma hominis and Ureaplasma urealyticum. Sexually transmitted diseases, 1986, 13:67–70.
- Judlin P. Mycoplasmes genitaux [Genital mycoplasmas]. Gynécologie, obstétrique & fertilité, 2003, 31(11):954–9.
- Witkin SS et al. Detection of Chlamydia trachomatis by the polymerase chain reaction in the cervices of women with acute salpingitis. American journal of obstetrics and gynecology, 1993, 168:1438–42.
- Lee AH et al. Molecular diagnosis of Ureaplasma urealyticum septic arthritis in a patient with hypogammaglobulinemia. Arthritis and rheumatism, 1992, 35:443–8.
- lanchard A et al. Evaluation of intraspecies genetic variation within the 16S rRNA gene of Mycoplasma hominis and detection by polymerase chain reaction. Journal of clinical microbiology, 1993, 31:1358–61.
- Arefi S et al. Anti-zona pellucida antibodies in follicular fluid and out come of ICSI. Middle East Fertility Society journal, 2006, 11(1):35–42.
- Skinner SM et al. Mapping of dominant b-cell epitopes of a human zona pellucida protein (ZP1). Biology of reproduction, 1999, 61:1373–80.
- Luborsky J et al. Ovarian autoimmunity: greater frequency of autoantibodies in premature menopause and unexplained infertility than in the general population. Clinical immunology, 1999, 90(3):368–74.
- Mhaskar A, Buckshee K, Talwar GP. Autoantibodies to zona pellucida in tuberctomized women. Contraception, 1984, 29(1):75–82.
- Nishimoto T et al. Autoantibodies to zona pellucida in infertile and aged women. Fertility and sterility, 1980, 34(6):552–6.
- Mantzavinos T et al Assessment of autoantibodies to the zona pellucida in serum and follicular fluid in in-vitro fertilization patients. Clinical and experimental obstetrics and gynecology, 1993, 20(2):111–5.
- 22. Dor J, Rudak E, Aitken R J. Antisperm antibodies: their effect on the process of fertilization studied in vitro. Fertility and sterility, 1981, 35:535–41.
- 23. Koch A et al. Mycoplasma hominis and Ureaplasma urealyticum in patients with sexually transmitted diseases. Wiener klinische Wochenschrift, 1997, 109:584–9.
- Kovacs GT et al. Microbiological profile of the cervix in 1,000 sexually active women. Australian and New Zealand journal of obstetrics & gynaecology, 1988, 28:216–20.
- Kapur TR et al. Isolation of ‘T’ mycoplasma in vaginal discharge. Journal of. obstetrics and gynecology of India, 1976, 26:879–83.
- Ynox CL, Timms P. Comparison of PCR, nested PCR, and random amplified polymorphic DNA PCR for detection and typing of Ureaplasma urealyticum in specimens from pregnant women. Journal of clinical microbiology, 1998, 36(10):3032–9.
- Young H, Tuach S, Bain SRS. Incidence of Ureaplasma urealyticum infection in women attending a clinic for sexually transmitted disease. Journal of infection, 1981, 3:258–65.
- Hill AC. Mycoplasmas, a review of surveys examining human genital infections and experimental infection in mice with special reference to in vitro fertilization. Lijecnicki vjesnik, 1990, 112:358–60.
- Mathur S et al. Antibodies to microbial, leukocyte and organ antigens. Journal of reproductive immunology, 1985, 8(4):353–8.
- Styler M, Shapiro SS. Mollicutes (mycoplasma) in infertility. Fertility and sterility, 1985, 44(1):1–12.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)