R. Hamkar,1 S. Jalilvand,1 M.H. Abdolbaghi,2 K.N. Jelyani,1 A. Esteghamati,3 A. Hagh-goo,4 T. Mohktari-Azad1 and R. Nategh1
التمييز بين العدوى الأولية وبين تجدُّد العدوى بفيروس لقاح الحصبة الألمانية عن طريق تحليل رغابة الغلوبولين المناعي G في الحوامل
رسول همكار، سمية جليلوند، محبوبة حاجي عبد الباقي، كرامت نوري جلياني، عبد الرضا استقامتي، آمنة حق كو، طلعت مختاري آزاد، رخشنده ناطق
الخلاصـة: أثناء الحملة الموسَّعة للتلقيح ضد الحصبة والحصبة الألمانية (الـحُمَيْراء) في شهر كانون الأول/ديسمبر 2003 التي جرت في جمهورية إيران الإسلامية، جرى تلقيح كثير من الحوامل عن طريق الخطأ، أو أنهن حَمَلْنَ خلال فتـرة أقل من شهر من تاريخ التلقيح. وللتمييز بين الحوامل اللاتي أُصِبْنَ بفيروس لقاح الحصبة الألمانية كعدوى أولية وبين اللواتي تجددت إصابتهن بالعدوى بفيروس الحصبة الألمانية بسبب اللقاح، جُمِعت عينات المصل من 812 حاملاً خلال شهر إلى ثلاثة أشهر بعد حملة التلقيح الموسَّعة. وتبين من تحليل رغَابَة الغلوبولين المناعي G أن 0.3% من النساء لم يكُن لديهنّ تفاعل الغلوبولين المناعي G النوعي للحصبة الألمانية؛ وأن 14.4% كان لديهنّ رغابة منخفضة للغلوبولين المناعي G المضاد للحصبة الألمانية ولذلك لم تكن لديهنّ مناعة ضد فيروس الحصبة الألمانية قبل التلقيح؛ وأن 85.3% كان لديهنّ رغابة مرتفعة للغلوبولين المناعي G ضد الحصبة الألمانية ويُعتَبَرن من حالات تجدّد العدوى.
ABSTRACT: During the mass measles/rubella vaccination campaign in 2003 in Iran, many pregnant women were vaccinated mistakenly or became pregnant within 1 month of vaccination. To distinguish pregnant women who were affected by rubella vaccine as primary infection from those who had rubella reinfection from the vaccine, serum samples were collected 1–3 months after the campaign from 812 pregnant women. IgG avidity assay showed that 0.3% of the women had no rubella-specific IgG response; 14.4% had low-avidity anti-rubella IgG and were therefore not immune to rubella before vaccination; 85.3% had high-avidity anti-rubella IgG and were regarded as cases of reinfection.
Distinction entre la primo-infection et la réinfection par le virus du vaccin contre la rubéole grâce au test d’avidité des IgG chez les femmes enceintes
RÉSUMÉ: Lors de la campagne de vaccination de masse contre la rougeole et la rubéole réalisée en 2003 en Iran, de nombreuses femmes enceintes ont été vaccinées par erreur ou se sont trouvées enceintes un mois après la vaccination. Afin de distinguer les cas de primo-infection par le vaccin contre la rubéole des cas de réinfection par ce vaccin, des échantillons de sérum ont été prélevés sur 812 femmes enceintes pendant une période comprise entre un et trois mois après la campagne. La mesure de l’avidité des IgG rubéoliques a montré que 0,3 % des femmes n’avaient pas de réponse ; 14,4 % avaient des IgG antirubéoliques de faible avidité et n’étaient donc pas immunisées avant la vaccination ; et 85,3 % avaient des IgG antirubéoliques de forte avidité et pouvaient donc être considérées comme des cas de réinfection.
1School of Public Health; 2Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to R. Hamkar:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
). 3Disease Management Centre, Ministry of Health and Medical Education, Tehran, Iran. 4Rasool Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
Received: 24/05/06; accepted: 27/07/06
EMHJ, 2009, 15(1): 94-103
Introduction
Rubella, if acquired during the first trimester of pregnancy, carries a 90% risk of congenital malformations for the fetus, called congenital rubella syndrome (CRS) [1]. Prevention of CRS is the main goal of rubella vaccination and 2 approaches are recommended [2]: prevention of CRS only through vaccination of adolescent girls and/or women of childbearing age, and elimination of both rubella and CRS through universal vaccination of infants with/without mass campaigns, surveillance and ensuring immunity in women of childbearing age [3].
There is a significant burden of disease globally as a result of CRS, and the World Health Organization (WHO) recommends that countries incorporate rubella vaccine into their vaccination programmes if possible [4–8].
Rubella vaccination in pregnancy caries a theoretical risk of CRS if the vaccine is administered during or just before pregnancy [3,9]. This is because the rubella vaccine is a live attenuated virus that is able to replicate in vaccinees and can cross the placenta to infect the fetus in about 2% of susceptible mothers. However, there is no evidence that fetal infection with the vaccine virus is harmful [9,10]. Prospective registries in several countries have identified no infants with CRS born to known seronegative women who received rubella vaccine within 3 months of conception; thus, the observed risk is zero [3]. Based on data from these registries, and using the upper 95% confidence limit of the binomial distribution, the maximum estimated risk is 0.6% among women vaccinated within 3 months of conception with the Cendehill or RA27/3 rubella strains, and the maximum estimated risk is 2.5% among susceptible women vaccinated within the first 2 months of pregnancy with the RA27/3 strain. These maximum theoretical risks remain lower than the 3% risk of a major congenital malformation in the general population. It has been suggested that there will always be an upper maximum theoretical risk greater than zero, no matter how large a study is carried out. It is believed, therefore, that vaccination in pregnancy can never be positively recommended [9,10].
During the mass campaign of measles/rubella (MR) vaccination in December 2003 in the Islamic Republic of Iran, a large number of pregnant women received MR vaccine mistakenly or became pregnant soon after vaccination. Based on existing data about the epidemiological features of rubella in the Islamic Republic of Iran, around 80%–90% of the above-mentioned women may have been immune against rubella before vaccination [11–13]. Therefore, they may have experienced a rubella reinfection by the vaccine strain, and a relatively small proportion of them (10%–20%) may have developed primary rubella infection from the vaccine strain. Although cases of CRS due to rubella reinfection, even by wild-type rubella virus, are very rare [14], it was seen as necessary to distinguish between pregnant women who were affected by rubella vaccine as primary infection and those who had rubella reinfection from the vaccine. Evidence of reinfection would be accepted if a patient who had pre-existing rubella antibodies showed a significant rise of IgG antibody titre or a rubella-specific IgM response, or both [1]. The IgM response is typically weak, but may sometimes be strong.
In this study, we used IgG avidity assay to determine the immune response among pregnant women receiving rubella vaccine in the mass vaccination campaign in the Islamic Republic of Iran in order to separate reinfection cases with high-avidity IgG antibodies from primary infections with low-avidity response. Despite the lack of any reports of CRS due to rubella vaccine, there is a theoretical risk of CRS. Therefore, susceptible women who experience primary infection from rubella vaccine should be followed up.
Methods
Background to the study
Prior to the mass vaccination programme extensive information about the contraindications to vaccination during pregnancy were released by the media and married women were advised at the time of vaccination about the risks of becoming pregnant for up to 1 month after vaccination. While 108 000 pregnant women did not participate in vaccination during the mass campaign, many pregnant women did receive the MR vaccine. Most were in the early days of pregnancy at the time of vaccination and therefore did not know about their pregnancy, while some women, against advice, became pregnant soon after vaccination. Following the mass campaign, 2 major hospitals in Tehran, Imam Khomeini and Rasool Akram hospitals, and our laboratory were prepared to advise pregnant women who had been inadvertently vaccinated. A total of 812 pregnant women were referred to the above centres and participated in the study.
Sample
The study sample was 812 pregnant women aged 15–25 years old [mean 21.9 (standard deviation 2.4) years] who had received MR vaccine mistakenly or became pregnant after vaccination. The MR vaccine contained measles Edmonston Zagreb strain and rubella RA27/3 strain (Serum Institute of India Ltd, Hadepsar, Pune, India). Serum samples were collected 1–3 months after the MR mass campaign in December 2003, and stored at –80 °C.
Commercial enzyme immunoassays
Serum samples were tested by both rubella-specific IgM and IgG enzyme-linked immunoassay (EIA). The differential assay of rubella high-avidity and low-avidity IgG antibodies can be used as a potent assay to distinguish between primary and secondary immune response. This assay is gaining popularity as a diagnostic method for the assessment of the time of infection [15–17]. However, the rubella IgM assay may not be an appropriate test to distinguish between the primary and secondary immune responses, because detection of rubella-specific IgM alone cannot be considered absolute proof of a recent primary infection. IgM response after primary infection may be prolonged, lasting up to several years. Furthermore, in some reinfections, rubella IgM is detectable [15].
The commercial kits used were the Enzygnost anti-rubella virus IgM and Enzygnost anti-rubella virus IgG (Dade Behring, Marburg, Germany). All assay protocols, cut-offs and interpretation of results were carried out according to the manufacturer’s instructions.
For the anti-rubella IgM assay 2 local (in-house) preparations of weak and strong positive IgM standards were included as external controls in every EIA run.
The avidity of IgG for rubella virus was measured by a protein-denaturing EIA where the antibodies were first allowed to bind to the rubella virus antigen, followed by elution by buffer with and without 35 mM diethylamine [15,18,19]. Each sample was tested at 2 replicates and a single serum dilution (1:200) was applied to each replicate. After incubation for 1 hour, test plates were washed 4 times, and then 1 replicate was soaked for 5 min in washing buffer and the other replicate for 5 min in washing buffer containing 35 mM diethylamine. Fresh buffers were applied and this step was carried out 2 more times. The plates were then washed 4 times with washing buffer. Then the test was continued according to the kit procedure. The remaining specific antibody was then detected using optical density (OD), and an avidity index (AI) was calculated as follows:
| AI (%)= | OD wells soaked with 35 mM | *100 |
| DEA | ||
| OD wells soaked with wash | ||
| buffer |
Four serum sample controls were used at each testing: strong high-avidity anti-rubella IgG antibody; moderate-avidity anti-rubella IgG antibody; low-avidity anti-rubella IgG antibody; and an anti-rubella IgG-negative serum sample.
The rubella IgG avidity cut-off point was 53% of that calculated previously in another study using well-defined panels of sera as primary and secondary immune response to rubella vaccine in order to determine low- and high-avidity rubella-specific IgG responses [15].
Statistical analysis
The parameters calculated included sensitivity, specificity and positive and negative predictive values along with corresponding 95% confidence intervals (CI) in the differential diagnosis of rubella primary and secondary immune responses for both rubella IgM EIA and rubella IgG avidity assay. The chi-squared test was used to determine the statistical difference between the parameters of the 2 measurements. The laboratory findings and personal data from study groups were also compared using the chi-squared test. The non-parametric Jonckheere–Terpstra statistical method [20] was employed to test for trend of avidity index values at the 1st, 2nd, 3rd and 4th months after vaccination. P-value < 0.05 was considered statistically significant.
Results
Rubella IgG avidity assay results
A total of 117 cases (14.4%) had low-avidity anti-rubella IgG and 2 cases (0.3%) did not have any rubella-specific IgG, while 693 cases (85.3%) had high-avidity anti-rubella IgG (Table 1).
The rate of low-avidity response was significantly related to age (Table 2) (P 20 years, and 88.0% exhibited high-avidity response to rubella vaccine.
Rubella IgM-EIA results
Rubella-specific IgM was detected in 90 cases (76.9%) of primary infection (low-avidity anti-rubella IgG), and not detected in 27 cases (23.1%) (Table 1). In the immune group, with high-avidity anti-rubella IgG, anti-rubella IgM was not detected in 691 cases (99.7%).
Comparison of rubella IgM-EIA with avidity assay
The rubella IgM-EIA was compared with the rubella IgG avidity assay. The sensitivity and specificity were determined using low-avidity anti-rubella IgG response (primary infection) and high-avidity anti-rubella IgG response (reinfection) cases (Table 1 and Table 3).
Effect of time of sample collection on rubella IgG avidity maturation
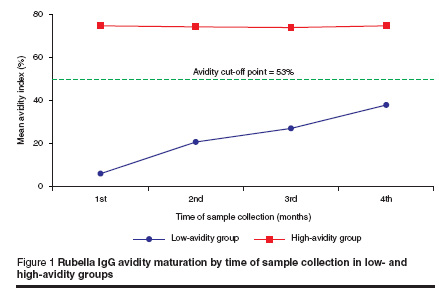
In the low-avidity IgG group, IgG avidity gradually increased over time; the mean avidity index was 6.12% in the 1st month, then increased to 20.82%, 27.16% and 38.03% in the 2nd, 3rd and 4th months respectively (Table 4 and Figure 1). This trend was highly significant in the low-avidity sera group (P < 0.001). However, our results showed no significant trend among avidity indices in the 1st, 2nd, 3rd and 4th months among the high-avidity sera group (P = 0.617).
Discussion
Rubella vaccination should be avoided in pregnancy because of the theoretical (but never demonstrated) teratogenic risk [10]. Data were available from the United States of America, the United Kingdom, Sweden and Germany on 680 live births to susceptible women who were vaccinated inadvertently 3 months before or during pregnancy with HPV-77, Cendehill or RA27/3 vaccines. None of the infants was born with CRS. However, a small theoretical risk of 0.5% (upper 95% CI = 0.5%) cannot be ruled out. Limiting the analysis to the 293 infants born to susceptible mothers vaccinated 1–2 weeks before conception or 4–6 weeks after conception, the maximum theoretical risk is 1.3% [9]. Although it is reassuring that no child was born with symptoms attributable to CRS, it is not appropriate to suggest that rubella vaccine is safe in early pregnancy [21].
According to existing data, about 80%–90% of fertile-age women are immune to rubella before vaccination [11–13]. Therefore, a relatively small proportion of inadvertently vaccinated pregnant women (10%–20%) may develop primary rubella infection from the vaccine strain. As documented reports of CRS by rubella reinfection, even by wild-type rubella virus, are very rare [14], distinction between primary infection and reinfection by the vaccine is necessary.
In the present study, IgG avidity assay was used to determine the immune response against rubella vaccine among pregnant women. The most critical part of this assay is the precise calculation of the cut-off point for differentiating low-avidity IgG from high-avidity IgG. A cut-off point equal to 53% of that previously calculated was applied to separate rubella low-avidity IgG from high-avidity IgG [15].
The laboratory findings indicated 14.7% of the women were not immune against rubella virus before vaccination, and 14.4% of them experienced a primary infection with the rubella vaccine strain. However, 85.3% of cases had high-avidity anti-rubella IgG, suggesting they were immune before vaccination and their response to rubella vaccine should be regarded as a secondary immune response. These results demonstrate that most women are immune at childbearing age. The findings also confirm previous reports of rubella immunity status in the Islamic Republic of Iran. The rate of immunity against rubella infection in our country was reported to range from 83% to 94.6% [11–13]. Thus around 5.4%–17% of Iranian women of childbearing age are susceptible to rubella infection, which means that there is a considerable risk of rubella infection during pregnancy, which could lead to CRS. According to these results, rubella vaccination was deemed necessary for elimination of CRS in the Islamic Republic of Iran. Mass vaccination in December 2003 provided appropriate immune coverage among women of childbearing age; only 0.3% of women failed to take up the vaccine. It is essential that vaccination against rubella enter into the policy of vaccination and that all of infants should be vaccinated against rubella for maintenance of this coverage.
Based on our results, the difference between primary infection and reinfection was statistically significant between the 2 age groups. Among the age group ≤ 20 years old, 21.4% experienced primary infection and 78.6% showed reinfection following rubella vaccination. However, among the > 20 years age group, 11.8% had primary infection and 88.2% experienced reinfection or no immune response from the rubella vaccine strain. This difference suggests that immunity against rubella infection increases with age.
Our sampling continued up to 4 months after vaccination and indicated that the avidity index values of the low-avidity group had an increasing trend by the time of sample collection, but were lower than the avidity cut-off point, even up to 4 months. In contrast, for the high-avidity group sera, the avidity index values were not affected by the timing of sampling: mean avidity indices were constant over the period of sample collection. So, according to these results, measurement of rubella-specific IgG avidity can distinguish primary infection from reinfection up to 4 months after vaccination. In one study it was observed that all samples had an avidity index 60%) was not observed until 13 weeks after infection.
In our study, rubella-specific IgM antibody was detected in 76.9% of sera containing low-avidity IgG, but 23.1% of sera tested by IgM-EIA provided negative results. While the sensitivity of IgM-EIA for diagnosing primary rubella infection was 76.9%, IgM-EIA did not have the appropriate sensitivity to distinguish between primary and secondary infections. However, IgM-EIA has appropriate specificity (99.7%) for determining negative cases and it can detect non-primary rubella cases, which again confirms previously reported findings [15]. Previously, the sensitivity and specificity of 7 commercial rubella-specific IgM kits were assessed, and it was shown that the sensitivity of most kits was within the range of 66.4%–78.9% (median 73.9%). The specificity of these kits was estimated to be 85.6%–96.1% (median 92.6%). The sensitivity and specificity of IgM-EIA for detection of rubella infection was shown by Behring’s indirect EIA kit to be 75.9% and 98.7%, respectively [5]. In another study it was shown that the percentage of rubella-specific IgM-positive sera decreased from 100% at 15–28 days after the onset of infection through 71%, 28% and 9% at 1–2, 2–3 and 3–4 months, respectively. After 4 months, all sera were negative for rubella specific IgM antibody. However, low-avidity specific IgG was detected in all of the sera taken at 3 months. At 3–4 months 91% and at 5–7 months 21% of sera still showed low-avidity [24].
Our study shows that the positive predictive value and negative predictive value of IgM-EIA were 97.6% and 96.4% respectively. When the prevalence of rubella is low, such as in countries with high rubella vaccination coverage, the positive predictive value of IgM testing decreases such that there is a significant risk of false positive results, and additional confirmation tests are therefore required [5,15,25]. The measurement of rubella-specific IgG avidity is a specific and sensitive method for the serological diagnosis of recent primary infection, and provides the distinction between primary infection and possible reinfection [15,18].
It is essential that a correct diagnosis of primary rubella can be achieved for the management of pregnant women with a recent rash or contact with a rubelliform rash illness [26]. With the introduction and widespread use of the rubella vaccine, it is likely that, with time, relatively few cases of rubella infection during pregnancy will be primary infection and more will be rubella reinfection [14,27]. Therefore, detection of IgM alone cannot differentiate primary infection from reinfection. For these cases, the measurement of IgG avidity is very useful, since, in the case of recent primary infection, IgG is low-avidity and in the case of reinfection, IgG is high-avidity [15,18,24,28,29].
References
- Best JM et al. Fetal infection after maternal reinfection with rubella: criteria for defining reinfection. British medical journal, 1998, 299:773–5.
- Control and prevention of rubella: evaluation and management of suspected outbreaks, rubella in pregnant women, and surveillance for congenital rubella syndrome. Morbidity and mortality weekly reports, 2001, 50(RR12):1–23.
- Report of a meeting on preventing congenital rubella syndrome: immunization strategies, surveillance needs. Geneva, 12–14 January 2000. Geneva, World Health Organization, 2000 (WHO/V&B/00.10).
- Cutts FT et al. Control of rubella and congenital rubella syndrome in developing countries. Part 1. Burden of disease from congenital rubella syndrome. Bulletin of the World Health Organization, 1997, 75(1):55–68.
- Robertson SE et al. Control of rubella and congenital rubella syndrome (CRS) in developing countries, Part 2: Vaccination against rubella. Bulletin of the World Health Organization, 1997, 75(1):69–80.
- Tipples G et al. Evaluation of rubella IgM enzyme immunoassays. Journal of clinical virology, 2004, 30(3):233–8.
- Rubella vaccines: WHO position paper. Weekly epidemiological record, 2000, 75(20):161–72.
- Preventing congenital rubella syndrome. Weekly epidemiological record, 2000, 75(36):290–5.
- Notice to readers: Revised ACIP recommendation for avoiding pregnancy after receiving a rubella-containing vaccine. Morbidity and mortality weekly reports, 2001, 50(49):1117.
- World Health Organization. Vaccination against rubella [website] (http://www.who.int/vaccines/en/rubella2.shtml, accessed 15 February 2008).
- Doroudchi M et al. Seroepidemiological survey of rubella immunity among three populations in Shiraz, Islamic Republic of Iran. Eastern Mediterranean health journal, 2001, 7(1–2):128–38.
- Ganjooie TA, Mohammadi MM. The prevalence of antibodies against rubella in pregnant women in Kerman, Iran. Saudi medical journal, 2003, 24(11):1270–1.
- Kabiri M, Moattari A. The rubella immunosurveillance of Iranian females: an indication of the emergence of rubella outbreak in Shiraz, Iran. Iranian journal of medical science, 1993, 18(3–4):134–7.
- Aboudy Y et al. Subclinical rubella reinfection during pregnancy followed by transmission of virus to the fetus. Journal of infection, 1997, 34:273–6.
- Hamkar R et al. Assessment of IgM enzyme immunoassay and IgG avidity assay for distinguishing between primary and secondary immune response to rubella vaccine. Journal of virological methods, 2005, 130(1–2):59–65.
- Bodéus M, Feyder S, Goubau P. Avidity of IgG antibodies distinguishes primary from non-primary cytomegalovirus infection in pregnant women. Clinical and diagnostic virology, 1998, 9:9–16.
- Korhonen MH et al. A new method with general diagnostic utility for the calculation of immunoglobulin G avidity. Clinical and diagnostic laboratory immuonology, 1999, 6(5):725–8.
- Gutiérrez J et al. Reliability of low-avidity IgG and of IgA in the diagnosis of primary infection by rubella virus with adaptation of a commercial test. Journal of clinical laboratory analysis, 1999, 13(1):1–4.
- Thomas HI, Morgan-Capner P. Rubella-specific IgG1 avidity: a comparison of methods. Journal of virological methods,1991, 31:219–28.
- Hollander M, Wolfe DA. Nonparametric statistical methods. Chapter 6. London, John Wiley, 1999:202–12.
- Tookey P. Pregnancy is a contraindication to rubella vaccination still [letter]. British medical journal, 2001, 322:1489.
- Hedman K et al. Maturation of immunoglobulin G avidity after rubella vaccination studied by an enzyme linked immunosorbent assay (avidity-ELISA) and by haemolysis typing. Journal of medical virology, 1989, 27(4):293–8.
- Bottiger B, Jensen PI. Maturation of rubella IgG avidity over time after acute rubella infection. Clinical and diagnostic virology, 1997, 8:105–11.
- Thomas HI et al. Persistence of specific IgM and low-avidity specific IgG1 following primary rubella. Journal of virological methods, 1992, 39:149–55.
- Best JM et al. Interpretation of rubella serology in pregnancy—pitfalls and problems. British medical journal, 2002, 325:147–8.
- Inouye S et al. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. Journal of clinical microbiology, 1984, 20(3):525–9.
- Thomas HIJ, Charlett A, Cubie HA. Specific IgG1 avidity maturation after rubella vaccination: A comparison with avidity maturation after primary infection with wild rubella virus. Serodiagnosis and immunotherapy in infectious disease, 1995, 7:75–80.
- Enders G, Knotek F. Rubella IgG total antibody avidity and IgG subclass-specific antibody avidity assay and their role in the differentiation between primary rubella and rubella reinfection. Infection, 1989, 17(4):218–26.
- Thomas HIJ, Morgan-Capner P. Rubella-specific IgG subclass avidity ELISA and its role in the differentiation between primary rubella and rubella reinfection. Epidemiology of infection, 1988, 101(3):591–8.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)