A.A. Abbassy,1 S.S. Barakat,1 M.M. Abd El Fattah,1 Z.N. Said2 and H.A. El Metwally3
هل بالإمكان الاستعاضة عن الخطة الحالية للتلقيح ضد الحصبة والحصبة الألمانية والنكاف بجرعة وحيدة من اللقاح المشتـرك بنهاية العام الأول؟
أحمد عمرو عباسي، شهيرة صلاح الدين بركات، محمد محمد عبد الفتاح، زينب نبيل سعيد، هالة عبد الرؤوف المتولي
الخلاصـة: تقدِّم هذه الدراسة المستعرضة تقييماً للحالة المناعية لدى أطفال أصحاء غير ملقَّحين، للتعرف على إمكانية الاستعاضة عن لقاحَيْن أحدهما هو المضاد للحصبة الذي يعطى في الشهر التاسع والثاني هو المضاد للحصبة والحصبة الألمانية والنكاف الذي يعطى في الشهر الثامن عشر، بجرعة وحيدة من اللقاح المضاد للحصبة والحصبة الألمانية والنكاف في الشهر الثاني عشر. وقد أظهرت عينات المصل المأخوذة من 566 طفلاً من الإسكندرية في مصر نقصاً يُعْتَدُّ به إحصائياً في معدَّل الإيجابية المصلية للأمراض الفيروسية الثلاثة مع تقدُّم العمر، رغم ازدياد يُعْتَدُّ به إحصائياً في معدَّل الإيجابية المصلية بين الأطفال الذين يحتلون مرتبة الطفل الأول أو الثاني في الأسرة، وللأطفال الذين ولدوا في تمام الحمل، أو من أمهات ليس لديهن سوابق ارتفاع ضغط الدم أثناء الحمل. ويوصي الباحثون بإعطاء الجرعة الأولى من لقاح الحصبة والحصبة الألمانية والنكاف بين الشهر التاسع والشهر الثاني عشر، ثم إعطاء جرعة معزِّزة منه في السنة الرابعة من العمر.
ABSTRACT: This cross-sectional study evaluated the immune status of non-vaccinated healthy infants to determine if it is possible to replace both measles vaccine (at 9 months) and measles, mumps and rubella (MMR) vaccine (at 18 months) with a single dose of MMR at 12 months. Serum samples from 566 children in Alexandria, Egypt showed a significant decrease in the seropositive rate to the 3 viral diseases with increasing age, but a significant increase in the seropositive rate among infants who were ranked 1st or 2nd in their family, full-term or born to mothers with no history of hypertension during pregnancy. We recommend administration of the first dose of MMR vaccine between 9 and 12 months of age, and a booster dose of MMR vaccine at 4 years of age.
Le calendrier vaccinal actuel (rougeole à 9 mois et ROR à 18 mois) peut-il être remplacé par une dose de ROR à 1 an en Égypte ?
RÉSUMÉ: Cette étude transversale a évalué le statut immunitaire de nourrissons en bonne santé non vaccinés afin de déterminer la possibilité de remplacer le calendrier vaccinal actuel, qui prévoit un vaccin antirougeoleux à 9 mois et un vaccin antirougeoleux-antiourlien-antirubéoleux (ROR) à 18 mois, par une dose unique de vaccin ROR à 12 mois. Les échantillons de sérum prélevés sur 566 enfants d’Alexandrie (Égypte) ont montré une baisse significative du taux de séropositivité aux trois maladies virales à mesure que l’âge augmentait, mais une augmentation significative de ce taux chez les nourrissons qui étaient le premier ou le deuxième enfant de la famille, nés à terme ou de mères n’ayant pas d’antécédents d’hypertension pendant la grossesse. Nous recommandons l’administration de la première dose de vaccin ROR entre 9 et 12 mois et une dose de rappel à l’âge de 4 ans.
1Department of Paediatrics; 3Department of Microbiology, Faculty of Medicine, University of Alexandria, Alexandria, Egypt (Correspondence to S.S. Barakat:
EMHJ, 2009, 15(1): 85-93
Introduction
The measles, mumps and rubella (MMR) viruses contribute to a significant degree of mortality and morbidity in developing countries [1]. Though MMR vaccine has been part of the immunization schedule in developed countries for some time, it has been included in the Egyptian national immunization schedule only since 2000. The MMR vaccine has been very effective in the elimination of disease and has high biosafety [2,3]. Furthermore, the vaccine can be safely administered to children with allergy to eggs, even those with severe hypersensitivity [4].
The Advisory Committee on Immunization Practices has recommended 2 doses of MMR vaccine for all children and certain high-risk groups of adolescents and adults, including international travellers, people attending colleges and other higher educational institutions and people who work at health care facilities. The first dose of MMR vaccine should be administered to all children beginning at or after age 12 months, and the second dose routinely at age 4 to 6 years [5]. More than 30% of measles cases occur before the age of 1 year in developing countries, a time at which that majority of children have lost their maternally-acquired antibodies [6]. The World Health Organization (WHO) recommended the vaccination schedule in Egypt as follow: measles vaccine at age 9 months and a single dose of MMR vaccine at 18 months.
Serological studies show that measles vaccine efficacy increases in children immunized at age 12 months or later [7,8]. This is due to the effect of persistent maternal antibodies that interfere with immunization [9,10]. Maternal antibody levels to measles show progressive reduction with increasing age from 7 months to 15 months [11]. This also applies to maternal mumps-specific IgG antibodies [12]. Gestational age also has an important influence on the placental transfer of maternal IgG antibodies to the fetus. The premature infant has proportionally lower IgG concentration at birth, and values reached their lowest level at 3 months [13]. However, seroconversion following vaccination is more common in premature than in term infants because of earlier disappearance of maternally-derived antibody in premature infants who start with a lower level of antibodies [14]. The immune status of mothers against the MMR viruses also has an important influence on the timing of immunization of children. It has been suggested that the children of women who have received measles vaccine might be successfully immunized at an earlier age than the children of women who have had measles. Antibody titres are lower after immunization than after measles, and therefore wane over a shorter period of time [14].
The aim of our study was to determine the immune status of non-vaccinated healthy infants in Egypt to ascertain if it is possible to replace the current vaccination schedule regarding measles (at 9 months) and MMR (at 18 months) by a single dose of MMR given at 12 months.
Methods
This cross-sectional study was carried out at the outpatient department of a tertiary care hospital in Egypt, Alexandria University Children’s Hospital, over a 1-year period from 1 March 2005 to 28 February 2006. A total of 566 normal children aged from 0–12 months, attending the hospital for regular follow-up, were enrolled in the study. Neonates less than 7 days were recruited from the delivery room. None of them had been previously vaccinated with MMR vaccine, had any history of measles, mumps or rubella infection or was taking immunosuppressive agents. Additionally, children with chronic illnesses such as malignancies, severe malnutrition, tuberculosis, liver cirrhosis or renal failure were not included in the study.
Written parental consent was obtained for each participant, and the study was approved by the ethics committee of the University of Alexandria. All studied infants were subjected to careful history taking (age, sex, birth order, gestational age, history of vaccination against any of the 3 viral diseases, maternal history of hypertension during pregnancy and maternal history of vaccination, infection or exposure to any of the 3 viral diseases) and full clinical examination.
A 3mL blood sample was withdrawn from each infant by venepuncture and sera were separated, divided in 3 aliquots and kept frozen at –70 ºC until tested for qualitative and quantitative measurement of serum levels of measles-, mumps- and rubella-specific IgG antibodies. Commercially available enzyme-linked immunosorbent assay kits were used for measles (BAG-Masern-EIA-G, BAG Healthcare, Lich, Germany), mumps (BAG-Mumps-EIA-G, BAG Healthcare) and rubella (Biotec Laboratories Limited, United Kingdom) [15]. Each test was performed following the manufacturer’s instructions. IgG levels were expressed as IU/mL.
Statistical analysis
The data were analysed using SPSS, version 5. Quantitative variables were presented as mean and standard deviation (SD). Analysis of variance (ANOVA) was used to compare the mean values of various quantitative variables between multiple groups. The chi-squared test was used to compare the proportion of quantitative variables between them. For all tests, a P value of < 0.05 was considered significant.
Results
The study included 566 healthy infants, aged between 0–12 months. They were 299 (52.8%) males. The studied subjects were categorized according to their ages into 5 groups: 0 day– 2– 4– 6– 9–< 12 months (58 infants, 10.2%)
Seropositive rates
The overall seropositive rate of the study infants to the 3 viral diseases was 68.0% for measles (385 infants), 79.5% for mumps (450 infants) and 57.6% for rubella (326 infants).
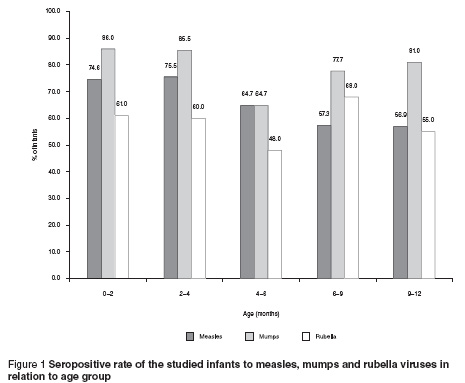
Changes in the seropositive rate of the studied infants to the 3 viral diseases in the different age groups are shown in Figure 1. The measles seropositive rate of the studied infants showed a significant decrease from 74.6% in the age group 0– 9–< 12 months (P 4– 6– 0.05).
Factors affecting seropositivity
Factors affecting the seropositive rate of the studied infants to the 3 viral diseases were as follows.
Gestational age
The median level of maternal-specific IgG antibodies to measles, mumps and rubella viruses was significantly higher in full-term compared to preterm infants (1.7, 113.5 and 23.1 IU/mL versus 0.0, 24.0 and 2.3 IU/mL respectively) (Table 1). Furthermore, the seropositive rate of the studied infants to measles, mumps and rubella was significantly higher in full-term compared to preterm infants (70.0%, 82.0% and 59.2% versus 0%, 0% and 5.9% respectively) (P < 0.001) (Table 1).
Birth order of the studied infants
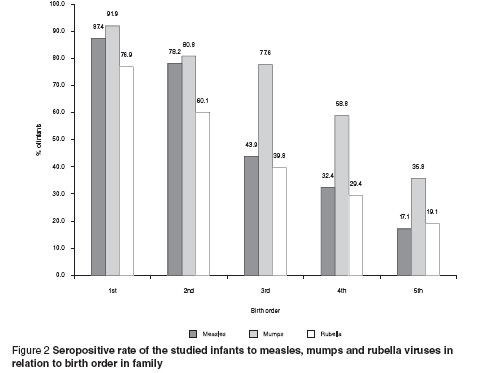
A higher seropositive rate to all the MMR viruses was shown among infants of 1st or 2nd birth order in their family, with a significant decrease in the percentage seropositive for infants ranked higher in the family birth order (P < 0.001) (Figure 2).
Maternal history of hypertension during pregnancy
We studied the effect of maternal hypertension during pregnancy on the median levels of maternal-specific IgG antibodies to MMR viruses and the corresponding seropositive rate among the studied infants. Infants born to mothers with hypertension during pregnancy showed a significant decrease in seropositivity to measles, mumps and rubella than those born to mothers with normal blood pressure during pregnancy (52.7%, 62.6% and 51.1% versus 72.6%, 84.6% and 59.5% respectively) (P 0.05) (Table 2).
Maternal history of exposure, infection or vaccination against the 3 viral diseases
We evaluated changes in the median levels of the maternal IgG antibodies to the MMR viruses among the studied infants in relation to maternal history of exposure, infection, vaccination or no history of infection with any of the 3 viral diseases. It was shown that the median levels of maternal IgG antibodies to measles, mumps and rubella were significantly higher among infants born to mothers with a history of previous infection to the corresponding virus (3.9, 233.2 and 406.7 IU/mL respectively) (Table 3). The highest seropositive rate to measles, mumps and rubella was found among infants born to mothers infected with the corresponding virus (49.1%, 42.6% and 31.9% respectively, not shown on Table 3).
Discussion
IgG antibodies are actively transported across the human placenta into the fetal circulation, supplying the infant with a full complement of its mother’s IgG antibodies. Such antibodies, which degrade over the first few months of life as the immune system of the infant matures, may protect against many infectious diseases but might interfere with the effectiveness of infant vaccination [16,17]. In this study we tried to evaluate the immune status of healthy, non-vaccinated Egyptian infants aged 0 day to 12 months to MMR viruses to assess if it is possible to change the current vaccination schedule regarding measles and MMR and replace it with a single dose of MMR given at 12 months.
The present study showed a decrease in the seropositive rate of the studied infants to the MMR viruses with increasing age. At the age of 9–12 months, about 50% of the studied infants still had the protective maternal antibodies, but also that at this age 50% of infants are susceptible to measles and rubella.
Variation in the prevalence of maternal antibodies to the MMR viruses between infant populations across countries and sociodemographic strata is poorly understood [9]. Some studies showed a persistence of maternal specific antibodies to measles in infants up to 15 months of age [11,18]. On the other hand, other studies showed early decay of maternal specific antibodies at age 6 months [10,19]. Several studies conducted at the University of Alexandria, Egypt showed a persistence of maternally transmitted antibodies to measles in only 30% of infants aged between 9–11 months, and to mumps in 86% of infants in the same age group [20–22]. Greater understanding of the determinants of the prevalence of maternal-specific antibodies will help national policy-makers to determine the appropriate age for infant vaccination for MMR [7]. The transfer of IgG from the mother to her fetus starts about the 6th month of gestation and increases sharply, so that fetal levels of IgG reach maternal levels during the 8th month of gestation. At term, the fetal level even exceeds the maternal level [17]. This implies that gestational age is an important determinant of transfer of IgG from the mother to the fetus [23]. The present study showed that significantly more full-term than preterm infants were antibody-positive to the MMR viruses. Also, the median levels of maternal-specific antibodies were significantly higher in full-term compared to preterm infants. These results agree with the work done by Okoko et al. [13] and Doroudchi et al. [24].
The birth order of the studied infants had an important impact on the seropositive rate to the MMR viruses, as infants ordered the 1st or 2nd had the highest percentage seropositivity compared with those ordered 4th or 5th. In Egypt, the poor nutritional state of multiparous mothers may affect their immune status, with a subsequent diminution of antibodies passively transferred to the fetus. A similar finding was observed in other studies [12,18].
Maternal diseases during pregnancy have an important impact on the rate of transmission of IgG antibodies across the placenta. The present study showed that the median levels of IgG titres and the proportion of infants who were antibody positive to the MMR viruses was higher among infants born to mothers who did not have a history of hypertension during pregnancy compared with those born to mothers with hypertension during pregnancy. This is might be attributed to ischaemic changes in the placenta that accompany hypertension and interfere with the normal transfer of maternal antibodies. Similarly, Sethi et al. found that IgG levels of low-birth-weight newborns (weighing less than 2 standard deviations below the expected weight for gestational age due to maternal illness during pregnancy) were lower than the antibody levels of normal weight infants of healthy mothers [25]. It is obvious that the decrease in serum IgG levels in infants born to mothers with antenatal illness is mainly due to the effects of maternal malnutrition and may possibly be related to the placental pathology commonly observed in these pregnancies [26].
Consideration has been given to the effects of maternal antibodies on the seropositive rate of the studied infants to the MMR viruses. The highest seropositive rate was found among infants born to mothers with a history of infection with any of the 3 viruses. Although our study did not include measuring maternal antibodies to the 3 diseases in the mothers of the studied infants, it is assumed to be highest among mothers with a history of infection. A study conducted in both developing and developed countries that measured maternal-specific antibodies to measles found that mothers who contracted measles had significantly higher levels of antibodies and higher seroprevalence rates among their infants than mothers who were vaccinated [26]. It has been suggested that the children of women who have received measles vaccine might be successfully immunized at an earlier age than the children of women who have had measles [14].
In conclusion, we recommend administration of the first dose of MMR vaccine between 9 and 12 months of age plus a booster dose of MMR at 4 years of age, according to the recommendations of the American Academy of Pediatrics [27].
Acknowledgements
This work received a grant from the Ministry of Health and Population. We acknowledge with thanks Professor Mohamed Ibrahim Kamel who did the statistical analysis of this manuscript.
References
- Burström B, Aaby P, Mutie DM. Measles in infants: a review of studies on incidence, vaccine efficacy and mortality in East Africa. East African medical journal, 1995, 72:155–61.
- Alyward RB, Clements J, Olive JM. The impact of immunization control activities on measles outbreaks in middle and low income countries. International journal of epidemiology, 1997, 26:662–69.
- Atkins GJ, Cosby SL. Is an improved measles-mumps-rubella vaccine necessary or feasible? Critical reviews in immunology, 2003, 23(4):323–38.
- James JM et al. Safe administration of the measles vaccine to children allergic to eggs. New England journal of medicine, 1995:332:126–6.
- Measles, mumps, and rubella. Vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and mortality weekly report, 1998, 47(RR-8):1–57.
- Sniadack DH et al. measles epidemiology and outbreak response immunization in a rural community in Peru. Bulletin of the World Health Organization, 1999, 77(7):545–52.
- Redd SC et al. Com Comparison of vaccination with measles-mumps-rubella vaccine at 9, 12, and 15 months of age. Journal of infectious diseases, 2004, 189(Suppl. 1):S116–22.
- Yadav S, Thukral R, Chakarvarti A. Comparative evaluation of measles, mumps, rubella vaccine at 9, 12, 15 months of age. Indian journal of medical research, 2003, 118:183–6.
- Caceres VM, Strebel PM, Sutter RW. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clinical infectious diseases, 2000, 31(1):110–9.
- Kebede S et al. Maternal rubella-specific antibody prevalence in Ethiopian infants. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2000, 94(3):333–40.
- Kiliç A et al. The duration of maternal measles antibodies in children. Journal of tropical pediatrics, 2003, 49(5):302–5.
- El Khayat HA et al. Serological status of mumps in non-immunized Egyptian infants. Scientific medical journal, 2003, 15(4):77–87.
- Okoko JB, Wesumperuma HL, Hart CA. The influence of prematurity and low birth weight on transplacental antibody transfer in a rural West African population. Tropical medicine and international health, 2001, 6(7):529–34.
- Yeager AS et al. Measles immunization: successes and failures. Journal of the American Medical Association, 1977, 237(4):347–51.
- Gerike E, Tischer A. In: Postmann T, ed. Diagnostische Bibliothek, Volume 1. Berlin, Blackwell Wissenschaftsverlag, 2000.
- Leach JL et al. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal–fetal antibody transport. Journal of immunology, 1996, 157:3317–22.
- Sidiropoulos D et al. Transplacental passage of intravenous immunoglobulin in the last trimester of pregnancy. Journal of pediatrics, 1986, 109:505–8.
- Eghafona NO et al. The levels of measles antibodies in Nigerian children aged 0–12 months and relationship with maternal parity. Epidemiology and infection, 1987, 99:547–50.
- Del Buono MB et al. Perdida con la edad de los anticuerpos maternos contra el sarampion en ninos de la ciudad de La Plata [Age-related loss of maternal antibodies against measles in children in La Plata]. Revista Argentina de microbiologia, 2003, 35(2):102–5.
- Aref GH et al. A study of antibody against measles in non-vaccinated infants and children [Master’s thesis]. Alexandria, Egypt, University of Alexandria, 1986.
- Aref GH et al. A study of antibody titer against mumps in non-vaccinated infants and children [Master’s thesis]. Alexandria, Egypt, University of Alexandria, 1986.
- Abbassy AA et al. Maternally transmitted antibodies against rubella in non-vaccinated infants. Alexandria journal of pediatrics, 1988, 2(3):309–11.
- Catty D, Drew R, Seger R. Transmission of IgG subclasses to the human fetus. In: Hemmings WA, ed. Protein transmission through living membrane. London, Elsevier, 1977:39–43.
- Doroudchi M et al. Placental transfer of rubella specific IgG in full term and preterm newborns. International journal of gynecology and obstetrics, 2003, 81(2):157–62.
- Sethi RS et al. Serum IgG levels in normal and low birth weight newborns. Indian pediatrics, 1980, 17:2–9.
- Brugha R et al. A study of maternally derived measles antibody in infants born to naturally infected and vaccinated women. Epidemiology and infection, 1996, 117:519–24.
- American Academy of Pediatrics. Immunization initiatives (http://www.cispimmunize.org/, accessed 1 December 2008).








