Y.S. Khader,1 Z.S.M. Albashaireh2 and M.M. Hammad3
حالة دواعم السن في السكريين من النمط الثاني مقارنة بغير السكريين في شمال الأردن
يوسف خضر، زكريا البشايره، محمد حماد
الخلاصـة: أجرى الباحثون في هذه الدراسة تقيـيماً لدواعم السن في 106 من السكريـين من النمط الثاني، وقارنوا النتائج مع 106 من غير السكريـين مما يضاهونهم في العمر. وقد جمعوا السكريـين من النمط الثاني ممن تزيد أعمارهم على 20 عاماً من العيادات الخارجية للطب الباطني في المستشفيَـين الرئيسيَـين في محافظة إربد في الأردن. وقد لوحظ أن مرض دواعم السن أشد لدى السكريـين من النمط الثاني مما هو عليه لدى غير السكريـين، ويدل على ذلك ارتفاع الـمَنْسَب اللثوي لديهم ارتفاعاً يُعْتَدُّ به إحصائياً، وعمق الجيوب في دواعم السن، ومستوى الارتباط السريري وحركية السن. ولم يكن هناك اختلاف يُعْتَدُّ به إحصائياً في متوسط مَنْسَب اللويحات السنية بين السكريـين وغير السكريـين. وكان مرض دواعم السن أكثر شدة بدرجة يُعْتَدُّ بها إحصائياً لدى السكريـين الذين تزيد فتـرة إصابتهم بالسكري على 5 سنوات منها عند الذين تقل فتـرة إصابتهم عن 5 سنوات.
ABSTRACT: The periodontal status of 106 type 2 diabetic patients was assessed and compared with that of 106 age-matched nondiabetics. Patients older than 20 years with type 2 diabetes mellitus were recruited from the outpatient internal medicine clinics at the 2 main hospitals in Irbid governorate, Jordan. Periodontal disease was more severe in type 2 diabetic patients than in nondiabetics, as indicated by significantly mean higher gingival index, periodontal pocket depth, clinical attachment level and tooth mobility. There was no significant difference in the mean plaque index between diabetics and nondiabetics. The severity of periodontal disease was significantly higher in patients with diabetes > 5 years than those with duration ≤ 5 years.
Comparaison de la santé parodontale de diabétiques de type 2 et de non-diabétiques dans le nord de la Jordanie
RÉSUMÉ: La santé parodontale de 106 diabétiques de type 2 a été évaluée et comparée à celle de 106 non-diabétiques appariés sur l’âge. Des patients âgés de plus de 20 ans et souffrant de diabète sucré de type 2 ont été recrutés dans les services de consultations externes de médecine interne des deux hôpitaux principaux du gouvernorat d’Irbid (Jordanie). Les parodontopathies étaient plus graves chez les diabétiques de type 2 que chez les non-diabétiques, comme le démontrent des valeurs significativement plus élevées en termes d’indice gingival moyen, de profondeur de poche(s) parodontale(s), de niveau d’attache clinique et de mobilité dentaire. En ce qui concerne l’indice de plaque moyen, il n’y avait pas de différence significative entre les diabétiques et les non-diabétiques. La parodontopathie présentait un niveau de gravité significativement plus élevé chez les patients atteints de diabète depuis plus de 5 ans que chez ceux atteints de diabète depuis 5 ans ou moins.
1Department of Community Medicine, Public Health and Family Medicine, Faculty of Medicine;
2Department of Restorative Dentistry, Faculty of Dentistry; 3Department of Preventive Dentistry, Faculty of Dentistry, Jordan University of Science and Technology, Irbid, Jordan (Correspondence to Z.S.M. Albashaireh:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
)
Received: 16/12/05; accepted: 23/02/06
EMHJ, 2008, 14(3):654-661
Introduction
Periodontal disease has been ranked 6th among the complications of diabetes mellitus [1] and is the most prevalent oral complication in patients with type 2 diabetes mellitus [2–4]. It has been found to be more common and more severe in diabetic patients than in controls [5–10]. The evidence of a direct relationship between periodontal disease and diabetes, gathered from thorough reviews, is strong [8,10]. Diabetes mellitus has been shown to be positively associated with clinical attachment loss [11]. A cross-sectional study of risk factors for periodontal disease in 1426 people found that diabetics had 2.32 times increased risk for attachment loss [12]. Diabetes affected all periodontal parameters, including bleeding scores, probing depths, loss of attachment and missing teeth [9].
It appears that diabetics have an increased susceptibility to periodontitis that is related to diabetes control [13] and duration of disease [14]. A substantial body of evidence has begun to emerge suggesting a bidirectional relationship between both types of diabetes and periodontal disease [15,16]. Nonsurgical periodontal treatment is associated with improved glycaemic control in type 2 diabetic patients and could be undertaken along with the standard measures for care of the diabetic patient [17]. Although this finding is intriguing, some reports showed that periodontal treatment had no effect on diabetes control [18,19].
As implicated in the literature, there may be a genetic component to type 2 diabetes. The relationship between diabetes and periodontal disease also appears to be very strong within certain populations, such as Aborigines [20,21]. Other factors are involved in the high prevalence of periodontal diseases in association with diabetes. A recent study found that smoking increases the risk of periodontal disease nearly 10-fold in diabetic patients [22]. Age is another factor, and researchers have documented that the differences between diabetic and control subjects with respect to periodontal disease may not be evident until the age of 30 to 40 years [23].
While diabetes mellitus is a common disease in Jordan with a prevalence of 13.4%, [24], no attempts have been made to explore the association between periodontal diseases and diabetes in Jordanian patients. This study was therefore conducted to assess the periodontal status of patients with type 2 diabetes mellitus attending outpatient clinics in Irbid compared with that of nondiabetics.
Methods
Sample
All consecutive patients older than 20 years with type 2 diabetes mellitus who attended the outpatient internal medicine clinics at the 2 main hospitals in Irbid governorate, Jordan, during a 4-month period in 2002 were included in the study. These central hospitals provide services to about 1 million inhabitants and the majority of diabetic patients are referred to them for specialized treatment. Simultaneously, another nondiabetic subject of the same or similar age was randomly selected and recruited from patients attending the orthopaedic and accident and emergency unit in the same hospital. Subjects were excluded if they had insulin-dependent diabetes mellitus, rheumatic arthritis, malignant blood disorders, allergy, asthma, or if they were pregnant or taking long-term medication other than diabetes therapy. Nondiabetics with a first sibling with diabetes were also excluded.
Informed consent for the interview and examinations were obtained from each participant in advance. The study was approved by the administration of the hospitals selected.
Data collection
The plaque index (PI) of Silness and Loe [25] was measured for 6 selected teeth, namely the maxillary right first molar, the maxillary right lateral incisor, the maxillary left first bicuspid, the mandibular left first molar, the mandibular left lateral incisor and the mandibular right first bicuspid. Missing teeth were not substituted. Thereafter, the periodontal status of all teeth excluding 3rd molars were assessed by the following parameters: gingival index (GI) of Loe and Silness [26], probing pocket depth (PPD), clinical attachment level (CAL), mobility of teeth using Miller mobility index, and number of missing teeth. All measurements were carried out on all participants.
Sterile dental mirrors and explorers were used to assess plaque accumulation and gingival conditions, while a standardized Michigan 0 periodontal probe with Williams’s markings (Diatech, Switzerland) were used to measure PPD and CAL. Mobility was assessed by applying pressure on the tooth in different directions using 2 hard instruments. Probing pocket depth was measured to the nearest millimetre from the gingival margin to the bottom of the crevice. CAL was measured to the nearest millimetre in cases of exposure of cement–enamel junction (CEJ) by reading off the distance from the CEJ or the margin of fixed restoration to the base of the pocket, and in other cases indirectly by subtracting the distance from the gingival margin to the CEJ from the pocket depth. The level of the CEJ was determined by feeling for it with the probe tip. Six (6) representative teeth and 4 surfaces of each studied tooth (mesiofacial, midfacial, distofacial and midlingual) were assessed and scored for PI. Other clinical parameters were collected at 6 sites per tooth for all teeth (mesiofacial, midfacial, distofacial, mesiolingual, midlingual and distolingual).
The average PI, GI, PPD, and CAL for each participant were computed by adding scores over all examined surfaces or sites and dividing by the total number of examined surfaces or sites. The average mobility score was computed over all examined teeth for each subject. The averages of these clinical parameters were used in the analysis.
All participants were interviewed for personal data including: age, sex, education, income, oral hygiene and smoking habit. Diagnosis and duration of diabetes were retrieved from the medical records of the patients. This was judged as a more reliable method as patients may not recall correctly the onset of the disease. The patients were classified according to the duration of diabetes as follows: ≤ 5 years and > 5 years.
All clinical examinations were carried out by 1 examiner, for which intraobserver reliability was determined in 20 participants by re-examining them on 2 subsequent days. Of the total number of duplicate PPD measurements, 96% were within ± 1 mm of each other and 89% fell within the same depth (exact agreement). Of the duplicate CAL measurements, 98% fell within ± 1 mm of each other and the exact agreement was 88%. Blinding the examiner to the health of the patient or their duration of diabetes was not possible.
Analysis
For a power of 80% and level of significance of 0.05, the sample size that would find a significant difference of 0.75 mm in the average CAL between the 2 groups, with a standard deviation of 1.8 was calculated as approximately 94 per group. The characteristics of participants by categorized demographic, oral hygiene and smoking variables were described using frequency distributions and analysed using the chi-squared test. The differences in plaque score and periodontal parameters between diabetics and nondiabetics were analysed using the Wilcoxon signed-rank test. Kruskal–Wallis H test was used to test the differences in clinical parameters between the 3 groups that were produced based on diabetic status and duration of diabetes. Pairwise multiple comparisons to test the differences between each pair were conducted by calculating the minimum significant difference (MSD) in mean ranks for pairwise comparisons according to the formula:
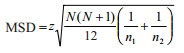
where N refers to the total sample size and n refers to the size of the specific group.
The analysis was done using SPSS, version 11.5. Comments about statistical significance refer to probabilities < 0.05.
Results
Characteristics of the study group
The present study involved 106 patients with diabetes mellitus (49 males and 57 females) and 106 people not suffering from diabetes (54 males and 52 females). The participants were age-matched; thus, the age distribution of diabetics was similar to that of nondiabetics. The characteristics of diabetic patients and nondiabetics according to demographic and oral hygiene variables are given in Table 1. Diabetic and nondiabetic groups had a similar distribution according to sex, level of education, smoking habit, brushing of teeth, use of auxiliary dental aids other than toothbrush (e.g. miswak, interdental brush and dental floss) and plaque index (Table 1). The proportion of diabetics with duration of disease since diagnosis ≤ 5 years was 52.8% and that of diabetics with duration > 5 years was 47.2%.
Effect of diabetes on periodontal status
Bivariate analysis demonstrated that there was no significant difference in the mean PI between diabetics and nondiabetics (P = 0.242). The mean GI, PPD, CAL, mobility score and number of missing teeth were significantly higher in diabetics compared with nondiabetics (Table 2).
Diabetics with disease duration > 5 years had significantly higher mean PI, PPD, CAL and mobility score than diabetics with disease duration ≤ 5 years (Table 3). The mean GI and mean number of missing teeth were not significantly different between the 2 diabetic groups. When the diabetics were compared with nondiabetics, both diabetic groups had a significantly higher mean PPD, CAL, mobility score and number of missing teeth. However, only diabetics with the disease duration > 5 years had higher mean GI than controls.
Discussion
This age-matched study was carried out to assess the periodontal status in a group of type 2 diabetes mellitus patients and compare it with that of a group of nondiabetics. Participants of this study were recruited from 2 referral hospitals which maintain appropriate records for their patients. Thus, the details of diabetic patients were extracted from their records as some may not recall the onset, duration or other details of their diabetes. The duration of diabetes was classified arbitrarily in 2 groups, ≤ 5 years and > 5 years, in order to make the methodology and the results of this study more comparable with those reported in the literature.
It must be emphasized that the results of this study may not be directly comparable with the results of others. This is due to many differences such as the population size, selection criteria for diabetic and nondiabetic groups, types of periodontal assessment performed, number of examiners, blinding of examiners and intra- and intersubject variations in measurements.
There was no significant difference in the average PI between diabetics and non-diabetics, a finding which contradicts some studies [6,9,27,28], but is accordance with another [2]. PPD and CAL were significantly greater in diabetics than nondiabetics, indicating that diabetics are at greater risk for developing periodontal disease than nondiabetics. These results are in accordance with some other reports [6,9,25]. The severity of periodontal disease was more prevalent in diabetics who had the disease for > 5 years. This finding is consistent with some reports [14,23] but not with another [27].
Although the exact role of diabetes in periodontal deterioration is still obscure, diabetes had been linked to increased susceptibility to periodontal disease through a number of hypotheses. Several interacting factors such as altered polymorphonuclear cell function and derangements of inflammatory protein response coverage at the periodontium result in a higher prevalence and severity of periodontitis [29]. Other factors, such as subgingival microflora and an alteration in host defences in diabetics may play a role in the association between periodontal disease and diabetes [30,31]. Grossi and Genco proposed a model for the biological association between periodontal disease and diabetes mellitus [15]. They mentioned that both the “infection-mediated” pathway of the periodontium and state of insulin resistance amplify the classical pathway of diabetic connective tissue destruction [advanced glycation end products (AGEs)-mediated].
The severity of diabetes and periodontal disease seem to be connected indirectly through health behaviours such as diet, frequency of meals, smoking and oral health behaviour [32]. It has been found that nonadherence with diabetes self-care instructions as a cause of poor metabolic balance was associated with not bothering to clean proximal surfaces as a cause of gingivitis [33]. Thus, while biological factors are certainly important, poor oral health behaviour leads to periodontal disease, and correspondingly poor adherence to diabetes metabolic control measures leads to complications including those related to the periodontium.
Because the severity of periodontal disease and tissue destruction of the periodontal apparatus may be accelerated as diabetes progresses, there is an obvious need for educational campaigns and intervention programmes for diabetic patients. Periodontal disease has to be managed and oral infections brought under control.
Conclusions
This study demonstrated that periodontal disease, as measured by mean GI, PPD, CAL and mobility scores, was more severe in diabetics than nondiabetics. It was also shown that diabetics had more missing teeth than nondiabetics. Diabetics with duration of diabetes > 5 years had significantly higher mean PI, PPD, CAL and mobility scores than in diabetics with disease duration ≤ 5 years.
References
- Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes care, 1993, 16:329–34.
- Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. Journal of periodontology, 1991, 62:123–31.
- Oliver RC, Tervonen T. Diabetes—a risk factor for periodontitis in adults? Journal of periodontology, 1994, 65:530–58.
- Yalda B, Offenbacher S, Collins JG. Diabetes as a modifier of periodontal disease expression. Periodontology 2000, 1994, 6:37–49.
- Cohen DW et al. Diabetes mellitus and periodontal disease: two year longitudinal observations. Part I. Journal of periodontology, 1970, 41:709–12.
- Sznajder N et al. Periodontal findings in diabetic and nondiabetic patients. Journal of periodontology, 1978, 49:445–8.
- Albrecht M, Bánóczy J, Tamás G Jr. Dental and oral symptoms of diabetes mellitus. Community dentistry and oral epidemiology, 1988, 16:378–80.
- Rees TD. The diabetic dental patient. Dental clinics of North America, 1994, 38:447–63.
- Bridges RB et al. Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control and socioeconomic factors. Journal of periodontology, 1996, 67:1185–92.
- Kinane DF, Chestnutt IG. Relationship of diabetes to periodontitis. Current opinion in periodontology, 1997, 4:29–34.
- Genco RJ. Current view of risk factors for periodontal disease. Journal of periodontology, 1996, 67:1041–9.
- Grossi SG et al. Assessment of risk for periodontal disease (I). Risk indicators for attachment loss. Journal of periodontology, 1994, 65:260–7.
- Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. Journal of clinical periodontology, 1993, 20:431–5.
- Belting CM, Hiniker JJ, Dummett CO. Influence of diabetes mellitus on the severity of periodontal disease. Journal of periodontology, 1964, 35:476–80.
- Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Annals of periodontology, 1998, 3:51–61.
- 16. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Annals of periodontology, 2001, 6:99–112.
- Kiran M et al. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. Journal of clinical periodontology, 2005, 32(3):266–72.
- Christgau M et al. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunologic results. Journal of clinical periodontology, 1998, 25(2):112–24.
- Janket SJ et al. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. Journal of dental research, 2005, 84(12):1154–9.
- Chen I. The Surgeon General’s report on oral health: implications for research and education. New York State dental journal, 2000, 66(9):38–42.
- Skrepcinski FB, Niendorff WJ. Periodontal disease in American Indians and Alaska Natives. Journal of public health dentistry, 2000, 60(Suppl. 1):261–6.
- Matthews DC. The relationship between diabetes and periodontal disease. Journal of the Canadian Dental Association, 2002, 68(3):161–4.
- Wolf J. Dental and periodontal conditions in diabetes mellitus: a clinical and radiographic study. Proceedings of the Finnish Dental Society, 1977, 73:1–56.
- Ajlouni K, Jaddou H, Batieha A. Diabetes and impaired glucose tolerance in Jordan: prevalence and associated risk factors. Journal of internal medicine, 1998, 244(4):317–23.
- Silness P, Loe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta odontologica scandinavica, 1964, 22:121–35.
- Loe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta odontologica scandinavica, 1963, 21:533–51.
- Faulconbridge AR et al. The dental status of a group of diabetic children. British dental journal, 1981, 151:253–5.
- Morton AA, Williams RW, Watts TLP. Initial study of periodontal status in non-insulin-dependent diabetics in Mauritius. Journal of dentistry, 1995, 23:343–5.
- McMullen J et al. Neutrophil chemotaxis in individuals with advanced periodontal disease and a genetic predisposition to diabetes mellitus. Journal of periodontology, 1981, 52:167–73.
- Offenbacher S. Periodontal disease: pathogenesis. Annals of periodontology, 1996, 1:821–78.
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontology 2000, 1997, 14:33–53.
- Moore PA. The diabetes–oral health connection. Compendium of continuing education in dentistry, 2002, 23(12 Suppl.):14–20.
- Kneckt MC, Syrjälä AM, Knuuttila ML. Attributions to dental and diabetes health outcomes. Journal of clinical periodontology, 2000, 27(3):205–11.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)