Harun-Ar-Rashid1
SUMMARY Health research in Bangladesh is increasing and hence there is a need to consider the ethical issues with regard such research. This paper describes the measures being taken in Bangladesh to address research ethics, such as the bioethics educational programmes and the ethics review committees functioning within the country. The role and work of the Central Ethics Review Committee and the regulatory guidelines are outlined. The paper also discusses the situation regarding research ethics within the South Asia region.
Perspectives régionales sur l’éthique de la recherche : rapport du Bangladesh
RÉSUMÉ La recherche en santé au Bangladesh s’accroît, d’où la nécessité de se pencher sur les questions éthiques concernant cette recherche. Le présent article décrit les mesures prises au Bangladesh pour aborder l’éthique de la recherche, telles que les programmes de formation dans le domaine de la bioéthique et les comités d’examen éthique en place dans le pays. Le rôle et les travaux du Comité central d’examen éthique ainsi que les lignes directrices réglementaires y sont présentés. L’article examine également la situation concernant l’éthique de la recherche dans la région de l’Asie du Sud.
1Director, Bangladesh Medical Research Council, Dhaka, Bangladesh (Correspondence to Harun-Ar-Rashid:
EMHJ, 2006, 12(Supplement 1): 66-72
Introduction
Bangladesh is a developing country of South Asia with enormous health problems. Demand for health research in this country is constantly increasing. International collaboration is growing rapidly. Bangladesh is getting the attention of international researchers because of the high prevalence of emerging and re-emerging infections, nutrition deficiencies, and family planning and reproductive health problems [1]. Under these circumstances Bangladesh needs a core group of highly trained professionals to provide guidance to the research community on ethical questions regarding the conduct of research, bearing in mind the current and future research situation.
The objective of this paper is to give an overview of the situation related to research ethics in Bangladesh.
Bioethics educational programmes
From 2003, the Bangladesh Medical Research Council has been conducting bioethics educational programmes under the International Bioethics Education and Career Development Award of the Fogarty International Centre of the National Institutes of Health, United States of America (USA). Under this education programme, which is called “Training on Research Bioethics” the BMRC conducts certificate and advanced courses on research bioethics. BMRC also organizes workshops on ethical issues in health research under its regular research programmes.
The objectives of the bioethics educational programmes are:
- To improve the ethical practice in conducting health research through capacity-strengthening of the health professionals/researchers of Bangladesh in research bioethics.
- To prepare a cohort of experts in Bangladesh in ethical review of research proposals who are capable of serving as members of the Ethics Review Committees.
The Training on Research Bioethics programme is supported by National Institutes of Health research grants and runs from 2003 to 2006. There are two components to this programme: the national component implemented in Bangladesh and the international component implemented in Kazakhstan.
Under the national component, two courses are conducted. One is the Certificate Course on Research Bioethics. The duration of the course is 10 weeks and two courses per year are to be conducted. Thus 6 such courses will be conducted over a period of three years. Each course includes 20 participants which will result in the manpower development of 120 professionals.
The second course is the Advanced Course on Research Bioethics. The course runs for 6 working days and one course will be conducted per year, giving a total of three courses over the three years. Our target is to train 30 health professionals (10 per course). Thus under this training programme, we are planning to train a total of 150 professionals in research ethics.
Under the Bangladesh Medical Research Council regular programme, workshops on ethical issues in health research were conducted during 1999–2000 and 2000–2001. These were held for 5 days and 28 health professionals were trained.
Under the Training on Research Bioethics programme we conducted two Certificate courses during 2003–2005 in which 78 participants were trained, and two Advanced courses in which 23 participants were trained. Thus a total of 101 health professionals have been trained in research bioethics through this training programme.
The distribution of participants for the Certificate course as regards institution is shown in Table 1 and illustrates that professionals working in the postgraduate institutions and medical colleges represented 55.1% of the total number trained. As regards profession and sex, 50% were public health experts or from the basic science and clinical departments and 55% were males.
Ethics review committees
At present in Bangladesh there is one central ethics review committee and nine institutional committees. The Bangladesh Medical Research Council’s Ethics Review Committee is considered as the Central/National Ethics Review Committee [2]. The nine other ethics review committees are functioning in 9 institutions. These committees are mostly attached to postgraduate medical institutes and there are two within two medical colleges. Of the nine, committees, five were recently formed. It is important to note that the participants who were trained through the Certificate and Advanced courses took the initiative to form these new ethics review committees in their own institutions. The nine committee are shown below (*recently formed ethics review committee).
- Bangabandhu Sheikh Mujib Medical University (BSMMU)*
- National Institute of Preventive & Social Medicine (NIPSOM)*
- National Institute of the Kidney Diseases and Urology, Dhaka*
- Institute of Child and Mother Health (ICMH)
- Bangladesh Institute of Child Health (BICH)
- Chittagong Medical College, Chittagong
- Sir Salimullah Medical College, Dhaka*
- Rajshahi Medical College, Rajshahi*
- Bangladesh Institute of Research for Promotion of Essential and Reproductive Health and Technologies
The International Centre for Diarrhoeal Diseases, Bangladesh (ICDDR’B) has its own Ethics Review Committee, which considers research studies submitted by the scientists of the Centre.
Central Ethics Review Committee and regulatory guidelines
The Central Ethics Review Committee of the Bangladesh Medical Research Council is considered the National Ethics Review Committee [2]. According to the Council’s policy, each and every project proposal approved by the Scientific Review Committee must also obtain the approval of the Ethics Review Committee before funding by the Council. This Committee provides approval for BMRC-funded research projects and also projects funded by organizations (both national and international) other than the BMRC, including multicentre collaborative studies and research studies leading to postgraduate degrees.
The Central Ethics Review Committee was established in 1979. The Committee consists of 9–11 members – lawyers, laypersons and religious leaders are included as members. The Committee is formed by the Executive Committee of the Bangladesh Medical Research Council under the guidelines of the Council for formation of the Ethics Review Committee and has a tenure of three years. The Committee is registered in the Office for Human Resource Protection in the USA as an official institutional review board and it has federal-wide assurance.
Every year, the Central Ethics Review Committee evaluates around 100 research proposals and it meets every month. The Committee follows certain strategies for approval of research proposals submitted for its consideration. The research proposals are grouped into three categories:
- Research studies involving non-invasive methods and interventions: The decision on these studies is taken on the basis of review by the members of the Committee.
- Research studies with invasive methods and/or interventions: These proposals require review of the ethical issues by technical experts. The number of reviewers ranges from 1 to 3 and depends on the nature of the research study.
- Research studies with policy implications: These studies are discussed through scientific meetings with relevant experts in addition to review of the ethical issues by technical experts.
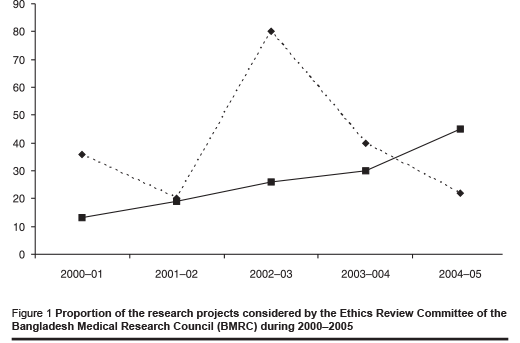
Figure 1 shows the percentage of the research projects considered by the Central Ethics Review Committee during the period 2000–2005. It shows that the research proposals funded by agencies other than the Bangladesh Medical Research Council are steadily increasing; these are mostly from international agencies. The Central Ethics Review Committee has developed an application form to handle such research proposals. The investigators apply using this form and should submit the umbrella proposal, the proposal summary, a summary of ethical issues involved, the informed consent form for subjects (this must be in Bengali), the consent form for parents or guardians, where applicable, the procedure for maintaining confidentiality and the questionnaire or interview schedule for consideration by the Committee.
The Central Ethics Review Committee has formulated a national policy on human tissue transplantation. BMRC has also developed guidelines for the transfer of human biomedical materials abroad for research purposes [2]. The Ethics Review Committee is in the process of developing national ethical guidelines for health research involving human subjects.
Regional perspectives
The countries of the South Asia region are in different stages of development with regards to health research and research ethics, and therefore their priority needs are different as regards the establishment of research ethics review systems.
The development of research ethics review systems in different countries of the region can be compared with Maslow’s law of hierarchy of needs (Figure 2) [3]. There are two basic stages of the law of hierarchy of needs: one is for survival and the other one is for achievement.
![Figure 2 Different stages of development with regards to health research ethics using the Maslow's hierarchy of needs model [3] Figure 2 Different stages of development with regards to health research ethics using the Maslow's hierarchy of needs model [3]](/images/stories/emhj/vol12/supplement-1/12-s1-12-f2.png)
There are countries in this region that are actually in the survival stage of development. They should ensure the basic needs – formation of a health research system, establishment of a medical/health research council or analogous body and subsequently an ethics review committee. Their next step is to ensure security needs, i.e. training of manpower, formation of a cohort of experts in research ethics, financial support and supply of an adequate number of research proposals for continuous functioning. It is important to note that there are countries where the numbers of sufficiently qualified technical experts are too limited for the development of an ethics review system. Development of the technical experts by training more experts and further taining of existing experts will enable the ethics review committee to function more correctly and effectively.
There are countries that have achieved the social needs. At this stage the ethics review committee should be recognized by others, the health professionals and the researchers. Firstly it should be recognized by the national bodies and then it should have some recognition outside the country – international recognition.
At the achievement stages, the next stage is fulfilling the ego needs. At this stage the ethics review committee should have developed its own guidelines and these guidelines should be used by other organizations, thus adding power to the ethics review committee. In Bangladesh, formulation of national ethical guidelines is underway. There are countries that have already formulated ethical guidelines and they have acquired the power. These countries are working to have the guidelines legally enacted so that they will be followed by others on the basis of legal standing. This will place the ethics review committee in the self-actualization stage of the hierarchy. The countries that are in the upper level, i.e. at the level of achievement in the hierarchy of research needs, and those that are at the lower level of survival should establish a good network so that both can benefit from available experts, committees and centres in the region.
Recommendations
Capacity-strengthening is recognized as a crucial step in the process of integrating the use of research for decision-making in the health system of a country [4]. Strengthened capacity in research ethics is needed in both developed and developing countries, although the need is particularly acute in developing countries. The crucial step, yet to be taken, is to strengthen ethics centres and training programmes in developing countries [5].
Capacity-strengthening in research ethics can be done through networking and it is applicable to institutions and also to individuals. Strengthening existing institutions through networking and setting up new institutions according to the needs of the country, and even the region, are essential. The establishment of the Center of Biomedical Ethics and Culture (CBEC) at the Sindh Institute of Urology and Transplantation in Karachi, Pakistan is a very positive development for strengthening research ethics review systems.
Training and continuing education, interaction at the regional and international level through organization of conferences, seminars and meetings are ways to strengthen ethics review systems. To maintain ethical standards in the countries of the region, the following actions are important: interaction between the regional ethics review committees, collaboration in review of ethical issues of research proposals and sharing of views in difficult situations. Formation of national ethical guidelines by the countries of the region and also enactment of these guidelines are crucial for further strengthening ethics review system in the countries of the region.
References
- Rashid HA. Research bioethics training in Bangladesh. Bangladesh Medical Research Council Bulletin, 2003, 29(3):78–85.
- Bangladesh Medical Research Council (BMRC). Organizational structure and functions of BMRC. Dhaka, Bangladesh Medical Research Council, 2003:8.
- DeCenzo DA, Robbins SP. Personnel – human resource management, 3rd ed. New Delhi, Prentice–Hall of India Private Limited, 1999:637.
- Rashid HA. Promoting road traffic injuries research in South Asia: capacity strengthening in health research. Journal of the College of Physicians and Surgeons--Pakistan, 2004, 14(12):736–8.
- Singer PA. Beyond Helsinki: a vision for global health ethics. Improving ethical behaviour depends on strengthening capacity. British medical journal, 2001, 322:747–8.








