P. Tabarsi,1 D. Mansouri,1 O Edrissian,1 A. Alaei,1 M. Amiri1 and S. M. Mirsaeidi1
1Department of Tuberculosis and HIV/AIDS, National Research Institute of Tuberculosis and Lung Diseases, Tehran, Islamic Republic of Iran (Correspondence to S.M. Mirsaedi:
Received: 30/05/04; accepted: 11/11/04
EMHJ, 2006, 12(6): 923-926
Case history
A 29-year old bisexual man was referred to the TB/HIV clinic in the Tuberculosis and HIV/AIDS Department, National Research Institute for Tuberculosis and Lung Diseases in Tehran, with a non-healing perianal ulcer and purulent discharge from the anus accompanied by episodes of fever, chills and generalized weakness. The patient’s complaint had begun 3 years previously and had been diagnosed as anal fissure in another hospital. He underwent the surgical removal of the fissure 2 times before being admitted to the clinic. Nevertheless, ulcer and discharge recurred. No histopathology results were available from those operations. During that period, he received various antibiotic regimens for his illness without improvement. He was found to be HIV positive 5 months before admission.
The patient is married and works as a welder. He had a positive history for opium abuse (opium smoking) and anal intercourse.
Physical examination revealed a moderate tenderness in the epigastric and right upper quadrant region. A perianal sinus, 5 cm × 2 cm with a depth of 4 cm, opening into the right side of the anus without extension to the anal sphincter was seen at the 3 o’clock position with the patient in the lithotomy position. The ulcer was surrounded by several follicles and covered by suppuration (Figure 1).
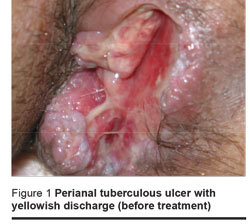
The patient’s tuberculin test was negative (induration < 5 mm). Laboratory tests showed hypochromic microcytic anaemia (haematocrit 32.8%; 45%–52% = normal range) as well as elevated levels of aspartate aminotransferase (90 IU/L; normal ≤ 46 IU/L), alanine aminotransferase (61 IU/L; normal ≤ 49 IU/L) and alkaline phosphatase (884 IU/L; normal 100–290 IU/L).
The absolute CD4+ T cell count was 598 (CD4+ 39%; lymphocytes 21%; white blood cell count 7300/mm3). Microscopic examination of sputum smear (repeated 3 times) was negative for Mycobacterium tuberculosis bacilli. The VDRL (Venereal Disease Research Laboratory) test was negative.
Biopsy of the ulcer was done by anuscope. Histopathologic examination of the ulcer showed chronic necrotizing granulomatous inflammation.
Microscopic examination of the specimen from the anal ulcer was positive for acid-fast bacilli and Mycobacterium tuberculosis was confirmed at the time by polymerase chain reaction to amplify polymorphic DNA regions flanking IS6110 by PCR with oligonucleotide primers to the end of IS6110. Specimens were cultured in Löwenstein–Jensen medium later. Mycological study of the specimen was negative. The chest X-ray was normal.
Abdominal sonography showed multiple lymphadenopathies in the para-aortic and mesenteric regions as well as the hilar portion of the liver. The abdominal computer tomography (CT) scan with oral and IV contrast confirmed the para-aortic and mesenteric adenopathies, and mild splenomegaly was seen as well. The CT scans of the brain and paranasal sinuses were normal. No abnormality was detected in colonoscopic and endoscopic evaluation of the patient. Needle biopsy from the liver was performed and the result was consistent with chronic granulomatous hepatitis. Microscopic study of the liver specimen was negative for acid-fast bacilli.
On diagnosis of the anal tuberculosis and granulomatous hepatitis in the patient, the standard 4-drug anti-tuberculosis regimen was begun as follows (patient’s weight was 63 kg): isoniazid 300 mg/day, pyrazinamide 1500 mg/day, rifampicin 600 mg/day and ethambutol 1000 mg/day [1].
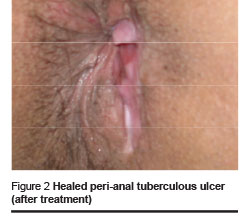
After 3 months, significant improvement was seen in the patient’s condition. The perianal ulcer had healed completely (Figure 2) and the liver enzymes decreased to normal levels. The patient is still under treatment and is being followed up as an outpatient.
Discussion
Involvement of the perianal region in tuberculosis is a rare extrapulmonary form of this disease. It comprises less than 10% of all perianal diseases and 0.7% of all tuberculosis cases [1]. In a report from India, 19 (15.6%) of 122 patients with anal fistula were diagnosed with tubercular fistula. Only 3 presented a clinical picture of concomitant pulmonary tuberculosis; none were HIV-positive [2]. In another report from Madagascar, 14% of 64 patients with extrapulmonary tuberculosis had tubercular anal fistula [3].
Diagnosis of perianal tuberculosis is difficult and needs a high suspicion index, especially in patients with perianal involvement as the first presentation of tuberculosis. Although extrapulmonary tuberculosis is common among HIV-positive patients [4], it seems that the incidence of perianal involvement is not increased by HIV infection [5].
Tuberculosis in the anal region presents as non-healing ulcer-like fissures, recurrent fistulas, perianal warty growths and abscesses [5,6]. Because of the absence of sufficient leading symptoms and signs, diagnosis of perianal tuberculosis can be much more complicated among HIV-positive patients. The diagnosis is confirmed after surgery and histological examination of excised tissue in most cases [2,7].
The case under discussion was the first HIV-positive patient with perianal tuberculosis in our clinic, the main referral centre for tuberculosis in the Islamic Republic of Iran. In this patient, lack of specific pulmonary symptoms, normal chest X-ray, negative tuberculin test and negative smear sputum were the confusing factors for diagnosing tuberculosis. In addition, no evidence of gastrointestinal involvement could be found. Delay in definite diagnosis of tuberculosis in this patient led to liver involvement that was demonstrated by an increase in serum alkaline phosphatase and aminotransferase levels compared to levels before starting treatment, and confirmed by liver biopsy soon after. It could have caused more severe complications if it had remained undiagnosed. Histologic examination of the lesion can be considered a valuable clue in the diagnosis of perianal tuberculosis. It revealed a disseminated infection or portal vein pathway for liver involvement. It is interesting that after 3 years duration there was no grave dissemination of M. tuberculosis in a patient with HIV.
Tuberculosis is the most common cause of death in HIV-positive patients in less developed countries [8]. Overlooking the diagnosis of perianal tuberculosis can result in high morbidity and mortality, especially among immunocompromised patients. In general, tuberculosis should be considered as one of the differential diagnoses in perianal lesions, specifically in recurrent ones. In such cases, thorough history taking, looking for acid-fast bacilli in the discharge from the lesion along with vigilant histological examination of excised tissue can lead to early diagnosis and treatment of the disease.
This report shows the importance of suspecting tuberculosis in all chronic lesions in HIV-positive patients.
References
- Logan VS. Anorectal tuberculosis. Proceedings of the Royal Society of Medicine, 1969, 62(12):1227–30.
- Shukla HS et al. Tubercular fistula in ano. British journal of surgery, 1988, 75(1):38–9.
- Ravolamanana Ralisata L, Rabenhamina FR, Ralison A. Les formes extra-thoraciques de la tuberculose en milieu hospitalier a Mahajanga (Madagascar [Extrathoracic forms of tuberculosis in Mahajanga hospitals (Madagascar)]. Archives de l’Institut Pasteur de Madagascar, 2000, 66(1–2):13–7.
- Harries AD, Maher D. TB/HIV a clinical manual. Geneva, World Health Organization, 1996:46–60.
- Sultan S et al. Anoperineal tuberculosis: diagnostic and management considerations in seven cases. Diseases of the colon and rectum, 2002, 45(3):407–10.
- Haas DW. Mycobacterium tuberculosis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, vol. 2, 5th ed. Philadelphia, Churchill Livingstone, 2000:2576–604.
- Candela F et al. Perianal disease of tuberculous origin: report of a case and review of the literature. Diseases of the colon and rectum, 1999, 42(1):110–2.
- Corbett EL et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Archives of internal medicine, 2003, 163(9):1009–21.








