M.M.S. Dallal,1 M.R. Khorramizadeh1 and K. MoezArdalan1
ABSTRACT This study was carried out on 1600 rectal swabs from children under 5 years of age admitted at the health centre in Islamshahr, Tehran province, Islamic Republic of Iran, during 1998–99. The specimens were examined for various bacterial pathogens. Isolation rates were: enteropathogenic Escherichia coli 6.8%, Shigella spp. 3.4%, Salmonella spp. 2.9%, Campylobacter spp. 0.9%, Yersinia spp. 0.7%. The isolation rate was highest in the summer, except for Yersinia spp., which was predominantly isolated in spring. The results of this study demonstrate the significance of Yersinia spp. and Campylobacter spp. in patients with diarrhoea.
Présence de bactéries entéropathogènes chez des enfants de moins de 5 ans souffrant de diarrhée dans le sud de Téhéran
RÉSUMÉ La présente étude a été réalisée sur 1600 écouvillonnages rectaux effectués chez des enfants de moins de 5 ans admis au centre de santé d’Islamshahr, province de Téhéran (République islamique d’Iran) en 1998-1999. Les prélèvements ont été examinés à la recherche de divers agents pathogènes bactériens. Les taux d’isolement étaient les suivants : Escherichia coli entéropathogène 6,8 %, Shigella spp. 3,4 %, Salmonella spp. 2,9 %, Campylobacter spp. 0,9 %, Yersinia spp. 0,7 %. Le taux d’isolement était plus élevé en été, sauf pour Yersinia spp. qui était isolé principalement au printemps. Les résultats de cette étude montrent l’importance de Yersinia spp. et de Campylobacter spp. chez les patients souffrant de diarrhée.
1Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to M.M.S. Dallal:
Received: 03/03/02; accepted: 22/06/03
EMHJ, 2006, 12(6): 798-803
Introduction
Diarrhoeal diseases constitute a major cause of morbidity in children. These diseases account for approximately 5–10 million deaths each year in Asia, Africa and Latin America, and are the major cause of death among 15%–20% of children under 5 years old. The annual child mortality rate reported by the World Health Organization is 120 million, of which 5 million are associated with diarrhoeal disease. Most deaths, however, occur in developing countries [1–3].
The infectious agents causing diarrhoea are usually transmitted by the faecal–oral route, i.e. through digestion of contaminated food, drinking contaminated water or direct contact with contaminated stool [4,5]. Diarrhoea is caused by a variety of infectious agents. Among the bacterial agents, 5 are important. These are Shigella spp., Salmonella spp., Escherichia coli, Yersinia spp. and Campylobacter spp. [6–10].
This is the first study from a poor suburban area of Tehran to evaluate the frequency of the major pathogens of childhood diarrhoea.
Methods
The study was conducted collaboratively with the health administration network of Islamshahr, a suburb with a population of about 300 000 in the south of Tehran, Islamic Republic of Iran. Rectal samples were taken from all children with diarrhoea under 5 years of age (1600 children) referring to the Health Centre of Islamshahr City in the one-year period June 1998 to June 1999. The samples were inoculated into Cary–Blair transport medium and were taken to the microbiology laboratory at the School of Public Health, Tehran University of Medical Sciences. Rectal swabs were used since the study was carried out in a relatively large geographic area where collection and transportation of fresh stool specimens were not feasible [11]. The specimens were checked microscopically (direct smear, Gram staining), then each specimen was cultured according to the standard method [11], with slight modifications as described.
In order to evaluate the role of the 5 major bacterial pathogens mentioned in the introduction, all specimens were cultured using Endo-agar (Merck 104044, Darmstadt, Germany), Salmonella–Shigella agar (Merck 107667), Yersinia selective agar (CIN) (Merck 116434), Yersinia selective supplement (Merck 116466), Campylobacter selective agar (Merck 102248) and Campylobacter selective supplement (Merck 102249). The cultures were incubated at an appropriate temperature.
The isolates were identified by biochemical and serological tests. For isolation of Salmonella spp., the specimens were inoculated in Selenite-F for 12 hours before primary culture. For isolation of Yersinia spp., the enrichment method (phosphate buffer saline, pH 7.0) with incubation at 4 °C was used (cold enrichment). After inoculation, the enrichment medium was incubated at 4 °C for 2 weeks, and then a loop of this was inoculated in CIN medium with supplement, and incubated for 2 further days at 25 °C. For isolation of Campylobacter spp., which has microaerophilic growth requirements, the gas pack system and anaerobic jar were used. After inoculation, the plates were incubated at 42 °C for 48 hours. Identification of suspicious colonies was then carried out according to standard criteria. Identification of enteropathogenic E. coli (EPEC) was done by slide agglutination with commercial antisera (bioMérieux, Marcy l’Etoile, France).
Results
Examination of the rectal swabs for 1600 children under 5 years of age showed that 235 (14.7%) children were harbouring 1 of the 5 major bacterial pathogens, i.e. enteropathogenic E. coli (EPEC) 109 (6.8%), Shigella spp. 54 (3.4%), Salmonella spp. 46 (2.9%), Campylobacter spp.15 (0.9%), and Yersinia spp. 11 (0.7%) (Figure 1).
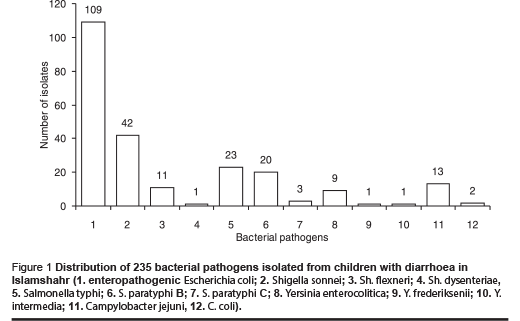
The isolation rate was much greater in males than in females, and frequency of the bacteria species isolated varied according to age group. For example, 67.0% of EPEC isolates were from children under 2 years old (serotypes: O26B6 42.2%, O55B5 24.8%, O119B14 13.8%, O127B8 9.2%, O111B4 6.4%, others 3.6%), whereas 25.9% of Shigella isolates and 21.7% of Salmonella were from children under 2 years (Table 1).
Three species of Shigella were identified in our study, Sh. sonnei, Sh. flexneri and Sh. dysenteriae, Sh. sonnei being the major isolate.
There was a high prevalence of S. typhi (group D), which contributed to 50.0% of total salmonellosis, and S. paratyphi B (group B) with a prevalence of 43.5%. Prevalence of S. paratyphi C (group C) was 6.5%.
Seasonal distribution showed that most of the cases of diarrhoea caused by E. coli and Shigella, Salmonella and Campylobacter species occurred in summer, probably owing to the increase in temperature, whereas most of the cases of diarrhoea caused by Y. enterocolitica occurred in spring (Table 2).
Discussion
Acute diarrhoea is a serious health problem for children under 5 years of age and also for adults [1,12]. The etiology of diarrhoea varies according to the geographic and climatic conditions. For example, in the cold areas of Europe, in contrast to the warm and tropical areas, Vibrio cholera, Aeromonas spp. and Plesiomonas shigelloides are seldom isolated, [3,6,12]. However, the prevalence of such psychrophilic bacteria as Yersinia in intestinal infections is higher in cold areas than in warm areas [13–15]. In the Islamic Republic of Iran, random studies have shown the contribution of bacterial etiologic agents to intestinal infections [16,17]. Our studies in Islamshahr showed that the 5 bacterial agents E. coli, Shigella spp., Salmonella spp., Campylobacter spp, and Yersinia spp. play an important role in gastroenteritis of children in this area, thus necessitating vigilance in maintaining environmental health indices.
From the diarrhoea specimens we examined, 14.7% were contaminated with one of the 5 causative bacterial agents. In a previous study done in 1993 in America, out of 4305 children with diarrhoea, 8.6% (372) were contaminated with one of the bacterial agents causing diarrhoea [14]. In another study carried out in New Caledonia in 1994, out of 2088 patients with diarrhoea, 5 bacterial pathogens were recovered in 587 (28%) cases [18].
The highest isolation rate (6.8%) was for EPEC. In a previous report made by Katouli et al. in which the frequency of EPEC serogroups was studied in 2 cities of the Islamic Republic of Iran, Tehran and Sanandaj [16]. The highest isolated serogroup in patients was O20ac (13.6%) in Tehran and O18ac (19.1%) in Sanandaj. More than 27% of EPEC isolates in our study were from children 1 year old or under, 67.0% from those under 2 years and more than 82% were from children under 3 years. The results show the significance of age in the etiology of gastroenteritis in children under 5 years.
Of the 3 species of Shigella identified in our study (Sh. dysenteriae, Sh. flexneri and Sh. sonnei, which belong to groups A, B, and D respectively), Sh. sonnei was the major isolate. No Sh. boydii (group C) was isolated. It has been reported that in recent years Sh. flexneri has been the dominating species in the Islamic Republic of Iran [16,19] but in our study, this species was less prevalent than Sh. sonnei.
Salmonella was the next most prevalent organism. The Salmonella strains we isolated belonged to 3 groups, B, C and D. There was a significantly high prevalence of S. typhi (group D).
The fourth most prevalent etiologic agent found in children’s diarrhoea in this study was Campylobacter. The morphology of this bacterium is quite different from the 4 others. They are all Gram-negative bacilli belonging to the Enterobacteriaceae, whereas Campylobacter belongs to the Spirillaceae. The prevalence of Campylobacter was a little higher (0.9%) than Yersinia (0.7%). All Campylobacter strains (15) isolated were from children under 2 years of age. We identified 86.7% as C. jejuni and 13.3% as C. coli.
Eleven specimens (0.7%) were diagnosed as Yersinia spp., Y. enterocolitica, Y. intermedia, and Y. fredriksenii. Two species, Y. intermedia and Y. fredriksenii, are usually isolated from contaminated waters. Thus, controlling the sanitary and hygiene conditions of water is very important. Out of the total positive cases for Yersinia spp. (11), 73% (8) were seen in winter and spring seasons. This finding shows agreement with previous studies [13,20].
Finally, further studies on the prevalence of such rare intestinal pathogens as Aeromonas hydrophila, Clostridium difficile, and various E. coli strains should be performed in other geographic areas to obtain more comparable epidemiological data.
Acknowledgements
We would like to express our appreciation to the local health centre in Islamshahr for their contributions and to Mrs Nazley Naimie from the microbiology laboratory at the School of Public Health, Tehran University of Medical Sciences for her technical assistance.
References
- Mathan VI. Diarrhoeal diseases. British medical bulletin, 1998, 54(2):407–19.
- Programme de lutte contre les maladies diarrhéiques : rapport intérimaire du programme 1992. Genève, Organisation mondiale de la Santé, 1993 (WHO/CDD/93.40).
- Lee WS. Gastrointestinal infections in children in the Southeast Asia region: emerging issues. Journal of pediatrics gastroenterology and nutrition, 2000, 30(3):241–5.
- Alamanos Y et al. A community waterborne outbreak of gastro-enteritis attributed to Shigella sonnei. Epidemiology and infection, 2000, 125(3):499–503.
- Gugnani HC. Some emerging food and water borne pathogens. Journal of communicable diseases, 1999, 31(2):65–72.
- Komathi AG, Ananthan S, Alfandi SV. Incidence and enteropathogenicity of Aeromonas spp. in children suffering from acute diarrhoea in Chennai. Indian journal of medical research, 1998:107:252–6.
- Huilan S et al. Etiology of acute diarrhoea among children in developing countries: a multicentre study in five countries. Bulletin of the World Health Organization, 1991, 69(5):549–55.
- Kotloff KL. Bacterial diarrhoeal pathogens. Advances in pediatric infectious diseases, 1999, 14:219–67.
- Yamashiro T et al. Etiological study of diarrhoeal patients in Vientiane, Lao People’s Democratic Republic. Journal of clinical microbiology, 1998, 36(8):2195–9.
- Pai M, Kang G, Ramakrishna BS. An epidemic of diarrhoea in south India caused by enteroaggregative Escherichia coli. Indian journal of medical research, 1997, 106:7–12.
- Manual for laboratory investigations of acute enteric infections. Geneva, World Health Organization, 1967 (Unpublished document WHO/CDD/83.3 Rev.1).
- Albert MJ et al. Case-control study of enteropathogens associated with childhood diarrhoea in Dhaka, Bangladesh. Journal of clinical microbiology, 1999, 37(11):3458–64.
- Phetsouvanh R, Midorikawa Y, Nakamura S. The seasonal variation in the microbial agents implicated in the etiology of diarrhoeal diseases among children in Lao People’s Democratic Republic. Southeast Asian journal of tropical medicine and public health, 1999, 30(2):319–23.
- Naqvi S et al. Presentation of Yersinia enterocolitica enteritis in children. Pediatric infectious disease journal, 1993, 12(5):386–9.
- Andersen J, Sorensen K, Glensbjerg M. Aspects of the epidemiology of Yersinia enterocolitica: a review. International journal of food microbiology, 1991, 13(3):231–8.
- Katouli M et al. Aetiological studies of diarrhoeal diseases in infants and young children in Iran. Journal of tropical medicine and hygiene 1990, 93(1):22–7.
- Katouli M et al. The role of Shigella spp. in childhood diarrhoea in Iran and their antibiotic resistance. Scandinavian journal of infectious diseases 1989, 21(4):415–9.
- Germani Y et al. Two-year study of endemic enteric pathogens associated with acute diarrhoea in New Caledonia. Journal of clinical microbiology, 1994, 32(6):1532–6.
- Behforouz NC, Amirhakimi GH, Gettner SM. Enteric pathogens in infants and children in Shiraz, Iran: A study of their incidences and infectious drug resistance. Pahlavi medical journal, 1977, 8(2):157–80.
- Abdel-Haq NM et al. Yersinia enterocolitica infection in children. Pediatric infectious disease journal, 2000, 19(10):954–8.








