A.A. Al-Ghoury,1 W.K. El-Hashimi2 and J. Abul-Hab3
ABSTRACT After the 1997–98 malaria epidemic in Babylon governorate, Iraq, malaria transmission in this area was successfully interrupted. A parasitological survey in 2002 identified no malaria cases but an entomological survey found both Anopheles stephensi and A. pulcherrimus in high densities. The highest density was recorded in September and the lowest in December and January. Despite the high density of Anopheles, no parasite sporozoites or oocysts were found in dissected mosquitoes. Nevertheless, malaria transmission could recur if A. stephensi indoor resting density exceeds the critical threshold and imported malaria cases are not monitored.
Épidémiologie du paludisme et prévisions concernant la reprise de la transmission dans le gouvernorat de Babylone (Iraq)
RÉSUMÉ Après l’épidémie de paludisme de 1997-1998 dans le gouvernorat de Babylone (Iraq), la transmission du paludisme dans cette région a pu être interrompue. Lors d’une enquête parasitologique en 2002, aucun cas de paludisme n’a été identifié mais une enquête entomologique a trouvé de fortes densités d’Anopheles stephensi et d’A. pulcherrimus. La densité la plus forte a été enregistrée en septembre et la plus faible en décembre et janvier. Malgré la forte densité d’anophèles, aucun sporozoïte ou oocyste n’a été trouvé dans les moustiques disséqués. Néanmoins, la transmission du paludisme pourrait reprendre si la densité de A. stephensi au repos à l’intérieur des habitations dépassait le seuil critique et si les cas de paludisme importés n’étaient pas suivis.
1Department of Parasitology, College of Medicine, University of Sana’a, Sana’a, Yemen (Correspondence to A.A. Al-Ghoury:
2Department of Microbiology; 3Department of Public Health, College of Medicine, Al-Mustansiriya University, Yarmouk, Baghdad, Iraq.
Received: 28/03/04; accepted: 06/07/04
EMHJ, 2006, 12(3-4): 270-279
Introduction
Malaria is considered one of the greatest challenges of all the health problems in tropical countries. The Plasmodium parasite undergoes a complete cycle of sporogony leading to the formation of the infective stages that propagate the infection during the feeding process of the mosquito. The efficiency of transmission of the disease depends mainly on the presence of favourable environmental conditions for the anopheline vectors [1]. Iraq has achieved a dramatic reduction in the number of malaria cases from an estimated 1 million per year in the early 1950s to less than 4000 cases per year by the 1990s.
Recently, the situation has been deteriorating in the 3 north-eastern governorates and malaria has spread outside this area [2]. Babylon governorate has been free of indigenous malaria cases since 1977, but a sudden epidemic occurred during the period 1997–98 in Hilla city, the capital of Babylon governorate [3]. The epidemiological features contributing to the epidemic included: increased rainfall and temperature in Babylon province after 1995 that favoured malaria transmission during that period; agricultural development projects around the city of Hilla that created new mosquito breeding habitats; and population movements when migrant workers returned from endemic areas in the north of Iraq. Control measures at the time included anti-plasmodial measures (treatment of cases, chemoprophylaxis and anti-relapse treatment, i.e. mass treatment); vector control (chemical and biological larvicides/insecticides and spraying with residual insecticides); individual protection aimed at reduction of man–vector contact; establishment of a malaria surveillance programme; and public health education.
The present study in 2002 aimed to evaluate the success of the interruption of malaria transmission after control measures at the focus of the 1997–98 epidemic. Parasitological and entomological criteria were used to predict the possible recurrence of transmission.
Methods
The study period was 1 year from 1 January to 31 December 2002.
A longitudinal parasitological and entomological survey was carried out in 2 districts: Centre district, which covers Hilla city, the capital of Babylon province and the focus of the 1997–98 epidemic, and the villages of Al-Hashimiyah district. Centre district includes 3 fixed stations, Al-Askery, Al-Zuweer and Offee, whereas Al- Hashimiyah district includes 3 fixed stations, Al-Owidine, Kisher and Al-Jebssah.
Parasitological survey
According to the World Health Organization there are 2 parasitological criteria indicating that transmission has been interrupted at a certain date. First, no resident child born after that date should ever be found positive. Second, careful surveillance with adequate coverage should detect no new cases after that date.
The sample was selected by multi-stage methods and the population of the study was then stratified into 5 groups as follows: malaria cases reported to the Primary Health Care Directorate during 1997–98 (n = 455), contacts of malaria cases (n = 795), children below 5 years of age from the same area (n = 314), foreigners arriving in the province (n = 24) and subjects from surrounding areas which had a high mosquito density (n = 97). The total for all groups was 1685 individuals. Table 1 indicates the age groups included. As both sexes are usually equally infected, sex was not included as a category.
Blood samples were collected from study subjects and all 1685 individuals were tested by ordinary thick and thin blood smears as this is regarded as the gold standard in malaria diagnosis. Thee other diagnostic methods were included to corroborate the smear method: haematocrit concentration (n = 352 individuals) [4]; improved thick blood films (n = 111) [5]; and rapid diagnostic (dipstick) test (n = 102) [6]. Financial constraints did not allow the same tests to be used on all samples. All thick and thin blood films were double checked by the general health laboratory of Primary Health Care Directorate in Babylon governorate.
Entomological survey
For the entomological survey, 6 fixed stations in the study areas were visited every month to carry out malaria vector surveillance in these areas. The vectors were monitored at both larval and adult stages from various habitats. The sucking tube collection method was used for outdoor and semi-indoor resting mosquitoes, but pyrethrum space spray was used for indoor resting mosquitoes.
In each station, 5 rooms were searched by 1 person who spent half an hour using an aspirator and a torch light. Also 5 rooms were searched by the pyrethrum space spray method in the morning [7]. In each station 10 suspected breeding places were searched and 10 dips were made in each place.
The samples of adult mosquitoes and larvae were examined and identified according to the keys described by Abul-Hab [8]. In addition, the salivary glands and stomach of the captured female mosquitoes were dissected for the detection of malaria sporozoites and oocysts [7].
Prediction of malaria epidemics
Entomological survey and indoor resting density were used for this purpose as well as entomological records before, during and after the 1997–98 epidemic. A. stephensi is the major malaria vector in central and southern Iraq [18], so that the indoor resting density of this species was used in a theoretical trial for predicting a malaria epidemic. Two methods were used to determine whether the household indoor resting density of A. stephensi has exceeded critical levels associated with epidemic transmission: the direct approach and the minimum sample size approach [9].
Statistical analysis
The results were presented in simple tables and graphs, using simple statistical measures as mean and variance as well as regression. P < 0.05 was considered statistically significant.
The following measures were calculated for the purpose of prediction of malaria epidemics:
1. Taylor’s power law, to measure the relationship between variance (s) and mean (x) as a power function [10].
![]()
parameters a and b are Taylor’s power law coefficients and provide information on insect aggregation.
2. An equation for calculating the number of houses required to estimate the mean Anopheles density for selected allowable errors using the direct approach [11].
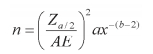
where Z a/2 = 1.96 at P = 0.05
n = number of houses required to calculate mean Anopheles density.
AE = allowable error, typically set between 10% and 50% of the mean.
3. An equation for calculating the number of houses required to estimate the mean Anopheles density using the minimum sample size approach [9].
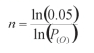
where 0.05 = error rate
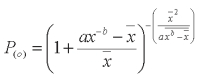
Results
Parasitological survey
A total of 2250 samples were tested from the 1685 individuals (Table 1). Although more than 1 diagnostic method was used in this study, no malaria cases were found using any of the 4 diagnostic tests. It is important to note that no cases were found among children below 5 years of age who were born after the epidemic of 1997–98.
Entomological survey
The entomological survey found that the 2 main malaria vectors in central and southern Iraq (A. stephensi and A. pulcherrimus) were present at the adult and larval stages.
The findings from all 6 fixed stations were grouped together according to the method of collection of the adults and larvae by month of collection (Figure 1). A. stephensi adults were present during all months of the year except January, whereas A. pulcherrimus adults were present only during May to November. The highest number of both A. stephensi and A. pulcherrimus adults were found in June and September/October respectively. The larval stages of A. stephensi disappeared in December and January when the mean temperature was below 14 ºC. A. pulcherrimus larvae appeared in May and continued to be present until November. Two peaks of the Anopheles larvae were seen: the first in June and the second in October.
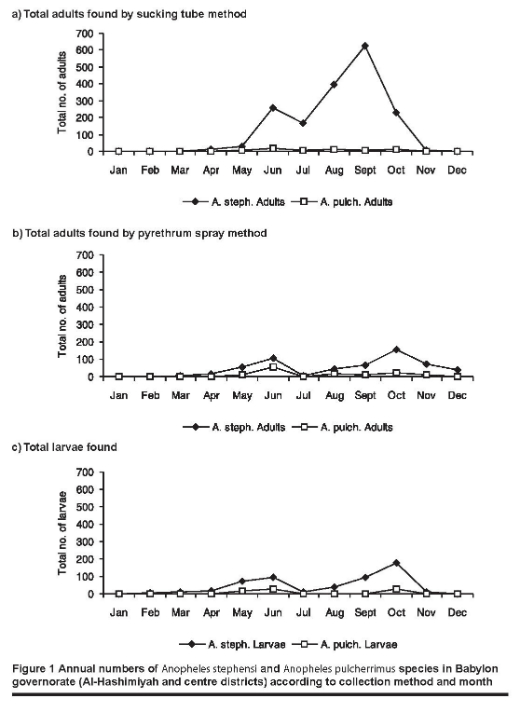
A small number (n = 85) of captured Anopheles adults were dissected for malaria oocysts and sporozoites. Neither oocysts nor sporozoites were found in either A. stephensi or A. pulcherrimus.
Prediction of malaria epidemics
The total number of A. stephensi caught in 6 stations from January to December 2002 was 1731 mosquitoes by pyrethrum spray collection method. This number was pooled into 1 sample, giving a mean 9.2 of mosquitoes per room (Table 2). The malaria annual reports in 1997–98 indicated that the epidemic began in October 1997 and peaked in January 1998 [3]. During the pre-epidemic months of August and September, the mean Anopheles density ranged from 0 to 11.2 mosquitoes per room. After the incidence of malaria began to decline in February, the mean Anopheles density ranged from 0 to 1.6 mosquitoes per room. An indoor resting density of 6 mosquitoes per room was chosen as the critical density indicating epidemic risk, because this value appeared to provide good separation between pre-and post-epidemic periods. Conversely, 1 mosquito per room was chosen as a normal density expected during non-epidemic periods. Because we were interested in periods of elevated Anopheles density, only those sampling periods with a mean Anopheles density > 1 mosquito per room were used to determine Taylor’s power law coefficients. The number of A. stephensi caught in 6 stations over 7 sampling periods (April to October 2002) were pooled into 1 sample with mean of 16.5 mosquitoes per room (95% confidence interval, 14.4–18.6). This was significantly greater than the mean Anopheles density in 1997–98, 8.1 mosquitoes per room.
Using the 7 sampling periods in which the mean A. stephensi density was > 1 mosquito per room, regression of [log (sampling period variance)] on [log (sampling period mean)] indicated a strong positive correlation (r = 0.98, P = 0.05). Estimates for the parameters a and b were 0.16 and 2.36 respectively, indicating aggregation in the data.
Direct approach
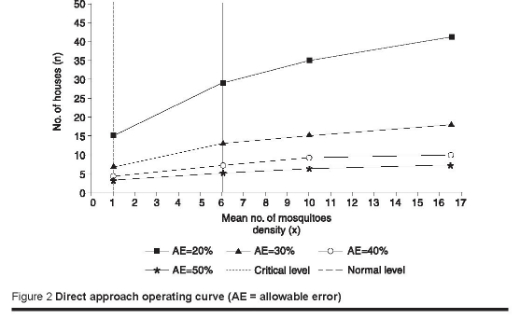
The number of houses required to calculate mean Anopheles density with allowable errors ranging from 10% to 50% of the mean was calculated using equation 2 with estimates of a and b as determined above. Samples of 115, 29, 13, 7 and 5 houses would be needed to calculate mean mosquito densities with allowable errors of 10%, 20%, 30%, 40%, and 50% respectively (Figure 2).
Minimum sample size approach
The number of houses required to find at least 1 A. stephensi according to the mean Anopheles density was calculated using equation 3 and the parameters a and b as determined above. Samples of only 1 house would be needed to calculate mean mosquito density with an error rate of 0.05%. Failure to find any mosquitoes in a sample of 1 house would indicate that Anopheles spp. density was not yet above the critical threshold.
Using the above 2 methods revealed that A. stephensi density had exceeded the critical threshold and indicated there was a risk of an epidemic (Figure 2).
Discussion
In the parasitological survey the total number of blood films examined was higher than other diagnostic methods, because this method is considered the gold standard in malaria diagnosis in Iraq and other countries [12,13]. Blood smears were collected from all age groups, but were carefully focused on children below 5 years of age who were born after the epidemic of 1997–98. All of their results were negative. The World Health Organization has 2 parasitological criteria indicating that transmission has been interrupted at a certain date. First, no resident child born after that date should ever be found positive. Second, careful surveillance with adequate coverage should detect no new cases after that date [14,15]. Similar studies in central and southern areas of Iraq [16] and in Nineveh governorate in 1998–2000 (G. Al-Mukhtar, in press) found that malaria transmission had been interrupted and was under control.
Northern Iraq belongs to the palaearctic region of the zoogeographical zones while its central and southern regions belong to the oriental zone and the annual occurrence of mosquito fauna differs accordingly between these regions. The annual occurrence of A. stephensi and A. pulcherrimus recorded by the entomological survey in the present study agree closely with the findings of Abul-Hab and Kassal [17] as well as Macan [18]. Our survey was extended to neighbouring districts from the original epidemic to check for the presence of the vectors. This is necessary now because Iraq is experiencing a re-emergence of the A. stephensi population after it had been well controlled since the 1960s [19].
It was found that the A. stephensi population was more abundant than A. pulcherrimus. A. stephensi is regarded as the only important vector of malaria in the central and southern regions [18]. There were 2 peaks of Anopheles species, larvae and adults, the first in June and the second in October. These findings agreed with the findings of Macan [18] and Abul-Hab and Kassal [17]. It is important to note that while both malaria oocysts and sporozoites were detected in these other studies, no such stages were found in the dissected specimens of either A. stephensi or A. pulcherrimus in this study.
Malaria transmission in Iraq is regarded as unstable in the whole country [20]. Therefore, it is vital to develop the tools to predict epidemics. In Iraq, increased Anopheles densities are not always associated with an epidemic but could be used as an indicator of epidemic risk. A. stephensi is the major malaria vector in the central and southern regions of Iraq. Indoor resting A. stephensi density was used as an indicator of epidemic risk when its density exceeded the critical level. Both the critical and normal thresholds were determined from the entomological data before, during and after the epidemic of 1997–98 [3]. We chose 6 mosquitoes per room as the critical threshold indicating the risk of an epidemic because it appeared to adequately separate mean A. stephensi density associated with epidemic transmission from the normal threshold during the post-epidemic period of 1997–98. Similarly, 1 mosquito per room was chosen as a normal threshold of A. stephensi density that was not associated with the risk of an epidemic. It was observed that closely similar thresholds of A. stephensi densities were found and recorded by the Communicable Disease Centre Baghdad, i.e. 5 mosquitoes per room was regarded as a critical level and below this value was regarded as a normal level [3]. In our study, both the direct approach and the minimum sample size approach were used for determining whether A. stephensi density had exceeded a critical level [9]. In the direct approach, it was demonstrated that calculation of mean Anopheles density with a high degree of precision requires a large number of houses, because of the variance in Anopheles density among houses. The number of houses required to sample would range from 5 to 115 houses for allowable errors of 50%–10% of the mean respectively. The mean A. stephensi density of 16.5 mosquitoes per room exceeded the critical level and indicated epidemic risk. In the minimum sample size approach, a mean density of 1 mosquito/house was required to determine whether A. stephensi density had exceeded the critical level. Less than this would indicate that the critical level had not been reached. However, it could be used as an initial screening tool during periods when Anopheles density was expected to be increasing. If a mean Anopheles density of 1 mosquito/house was found, monitoring could continue using the direct approach. A similar study in the African Highlands [9] found that it is feasible and probably appropriate to include monitoring of Anopheles spp. density in the prediction of malaria epidemics.
This study concludes that malaria transmission in the Babylon governorate was successfully interrupted after the recent epidemic of 1997–98. However, malaria transmission could recur if A. stephensi indoor resting density exceeds the critical threshold and imported malaria cases are not monitored. We propose that malaria epidemics should be predicted by measuring the indoor resting mosquito density.
Acknowledgements
We thank Dr Jalil Al-Zudaidi, Director of Endemic Diseases Institute, Baghdad, for his assistance and all the staff of the malaria unit Primary Health Care Directorate, Babylon Governorate for their continuous help during most stages of the fieldwork of this study.
References
- Radford A J, Van Leeuwen H, Christian SH. Social aspects in the changing epidemiology of malaria in the highlands of New Guinea. Annals of tropical medicine and parasitology, 1976, 70:11–23.
- Roll back malaria. Epidemiological situation. Iraq. Online fact sheet. Cairo, World Health Organization Eastern Mediterranean Regional Office (http://www.emro.who.int/rbm/EpidemiologicalSituation-CountryProfiles.htm, accessed 30 October 2005).
- Annual reports of malaria in Iraq. Baghdad, Iraq, Communicable Disease Centre, Ministry of Health, 1997–98.
- Worth MR. The heparinized capillary tube as an epidemiologic tool. II. concentration of blood parasites by centrifugation. American journal of hygiene, 1964, 80:70–4.
- Al-Khairy KS. An improved method of preparing thick blood films for the examination of malaria parasites. Saudi medical journal, 1992, 13:542–5.
- Malaria diagnosis—new perspectives. Report of a joint WHO/USAID informal consultation, 25–27 October 1999. Geneva, World Health Organization, 2000 (WHO/CDS/RBM/2000).
- Manual on practical entomology in malaria prepared by the WHO division of malaria and other parasitic diseases. Geneva, World Health Organization, 1975.
- Abul-Hab J. Medical and veterinary entomology in Iraq. Baghdad, Iraq, Baghdad University, 1979.
- Lindblade KA, Walker ED, Wilson ML. Early warning of malaria epidemics in African highlands using Anopheles (Diptera: Culicidae) indoor resting density. Journal of medical entomology, 2000, 37: 664–74.
- Taylor LR. Aggregation, variance and the mean. Nature, 1961, 189:732–5.
- Wilson LT, Room PM. Clumping patterns of fruit and arthropods in cotton, with implications for binomial sampling. Environmental entomology, 1983, 12:50–4.
- Ossi GT. Malaria in Iraq from 1980 to 1983. Bulletin of endemic diseases, 1984, 24:5–23.
- Gilles HM, Warrell DA, eds. Bruce-Chwatt’s essential malariology, 3rd ed. London, Edward Arnold, 1993.
- WHO Expert Committee on Malaria [meeting held in Geneva from 21 to 27 September 1965]: twelfth report. World Health Organization, Geneva, 1966 (Technical Report Series, No. 324).
- WHO Expert Committee on Malaria [meeting held in Geneva from 19 to 30 October 1970]: fifteenth report. Geneva World Health Organization, 1971 (Technical Report Series, No. 467).
- Shihab KI et al. Immunological and parasitological survey in areas of Iraq where malaria transmission has been interrupted since several years. Bulletin of endemic diseases, 1987, 28:17–28.
- Abul-Hab J, Kassal, S. Impact of anti-malaria spraying on the occurrence of Anopheles (Diptera: Culicidae) in Iraq. Bulletin of endemic diseases, 1986, 27:37–51.
- Macan TT. The anopheline mosquitoes of Iraq and North Persia. In: Leeson HS et al., eds. Anopheles and malaria in the Near East (Memoirs of the London School of Hygiene and Tropical Medicine, No. 7). London, HK Lewis, 1950.
- Annual reports of malaria in Iraq. Baghdad, Iraq, Communicable Disease Centre, Ministry of Health, 1960–2001.
- Al-Kafajei A, Ahmed KJ. Effectiveness of malaria control programs in Nineveh 1992. Journal of community medicine, Baghdad, 1993, 6:135–42.








