A. Azarkeivan,1 M. Hashemieh,2 S. Akhlaghpoor,3 A. Shirkavand,4 M. Yaseri 5 and K. Sheibani 6
العلاقة بين فيرّيتين المصل والتصوير بالرنين المغناطيسي T2 للكبد والقلب في مرضى الثلاسيميا الكبرى
آزیتا آذرکیوان، مزقان هاشميه، شهرام اخلاق بور، افشان شیرکوند، مهدي ياسري، كوروش شيباني
الخلاصـة: تدعو الحاجة إلى طريقة غير جارحة وذات دقة عالية لتقدير كمية الحديد العضوي لدى مرضى الثلاسيميا. وفي هذه الدراسة تقييم للعلاقة بين مستوى الفيرّيتين في المصل ومستويات الإنزيمات الكبدية، وضد الالتهاب الكبدي "سي" وترسبات الحديد في الكبد وفي القلب بقياسها بالتصوير بالرنين المغناطيسي T2. وقد حصل الباحثون على المعطيات من السجلات الطبية لـ 156 مريضاً بالثلاسيميا الكبرى في طهران، واتضح وجود تَرَابُط سلبي متوسط الشدة بين مستوى الفيرّيتين في المصل وبين التصوير بالرنين المغناطيسي بوقت الارتخاء T2 للكبد (r = -0.535)، وترابُط سلبي ضعيف بين الفريتين وبين التصوير بالرنين المغناطيسي بوقت الارتخاء T2 للقلب (r = -0.361). ولا تؤدي العدوى بالالتهاب الكبدي "سي" ولا مستويات الإنزيمات الكبدية إلى إرباك أو تعديل العلاقة بين الفيرّيتين وبين التصوير بالرنين المغناطيسي T2 للكبد أو للقلب. وكانت قراءات التصوير بالرنين المغناطيسي T2 للكبد وللقلب ذات تَرَابُط ضعيف (r = 0.281). ويقترح الباحثون إجراء تقييم روتيني لمحتوى الكبد والقلب من الحديد باستخدام التصوير بالرنين المغناطيسي T2 من أجل الوصول إلى تقييم أفضل لحالة ترسب الحديد والهيموسيديرين في مرض الثلاسيميا.
ABSTRACT There is a need for higly accurate non-invasive methods for assessing organ iron content in thalassaemia patients. This study evaluated the relation between serum ferritin level, liver enzyme levels and hepatitis C antibody and liver and heart iron deposition assessed by MRI T2*. Data were obtained from the medical records of 156 thalassemia major patients in Tehran. There was a moderate negative correlation between serum ferritin and liver MRI T2* relaxation time (r = –0.535) and a weak negative correlation between serum ferritin and heart MRI T2* relaxation time (r = –0.361). Hepatitis C infection and liver enzyme levels did not confound or modify the relation between ferritin and liver or heart MRI T2*. Liver and heart MRI T2* readings were poorly correlated (r = 0. 281). Routine evaluation of liver and heart iron content using MRI T2* is suggested to better evaluate the haemosiderosis status in thalassemia patients.
Relation entre la ferritine sérique et l'IRM hépatique et cardiaque pondérée en T2* chez des patients atteints de bêta-thalassémie majeure
RÉSUMÉ Des méthodes non-invasives de haute précision sont nécessaires pour l'évaluation de la concentration en fer dans les organes des patients atteints de thalassémie. La présente étude a évalué la relation entre le taux de ferritine sérique, les taux d'enzymes hépatiques, la présence d'anticorps anti-hépatite C et les dépôts de fer dans le foie et le cœur examinés par IRM pondérée en T2*. Les données ont été obtenues à partir des dossiers médicaux de 156 patients atteints de thalassémie majeure à Téhéran. On a observé une corrélation négative modérée entre la ferritine sérique et le temps de relaxation T2* de l'IRM hépatique (r = –0,535) ainsi qu'une faible corrélation négative entre la ferritine sérique et le temps de relaxation T2* de l'IRM cardiaque (r = –0,361). L'infection par le virus de l'hépatite C et les taux d'enzymes hépatiques ne constituaient pas de facteurs de confusion ou de modification de la relation entre la ferritine et l'IRM hépatique ou cardiaque pondérée en T2*. La corrélation entre les résultats de l'IRM hépatique et de l'IRM cardiaque pondérées en T2* était médiocre (r = 0,281). L'évaluation systématique de la concentration de fer dans le foie et le cœur à l'aide de l'IRM pondérée en T2* semble produire une meilleure évaluation de la présence d'une hémosidérose chez les patients atteints de thalassémie.
1Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Islamic Republic of Iran. 2Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to M. Hashemieh:
Received: 15/09/11; 03/06/12
EMHJ, 2013, 19(8):727-732
Introduction
Beta thalassaemia syndromes are the most common inherited haemoglobinopathies caused by a genetic deficiency in the beta-globin chain synthesis [1].
Although regular blood transfusion has increased the survival rate among thalassaemia patients, they develop severe cardiopulmonary, liver, endocrine, and other major organ dysfunctions due to iron overload [2]. The increased iron deposition coming from multiple life-long transfusions and enhanced iron absorption leads to organ dysfunction [3,4].
Estimation of iron deposits in different organs of thalassaemia patients is an important aspect of their medical management [5]. Serum ferritin has been used as a surrogate marker, but it is not specific because its level can be raised in inflammations such as hepatitis, infections and in liver damage [6]. Although biopsy is the most reliable method for estimating organ iron overload, its invasive nature makes its implementation very limited, and its accuracy is greatly affected by hepatic inflammation, fibrosis and uneven iron distribution [6]. The limitations of serum ferritin and liver biopsy make the search for non-invasive methods, with high accuracy, for assessing the organs iron content a necessity.
The T2* magnetic resonance imaging (MRI) technique is a non-invasive, valid and reproducible method for assessing tissue iron loading, and therefore has important implications for clinical management of iron overload and the tailoring of chelation regimes [7–10]. This method has allowed for early diagnosis of liver [7,8] and heart [11,12] haemosiderosis and a reduction in morbidity and mortality in thalassaemia patients [13].
Cardiac failure is the leading cause of death among thalassaemia patients [14]. Hepatic iron concentrations, which have traditionally been used as a representation of total body iron load, do not directly correlate with other organ iron concentrations, as the liver and other tissues such as cardiac or renal tissue have different mechanisms and kinetics of iron uptake, storage and clearance [15–17]. When comparing the liver and heart MRI T2* readings among patients, different degrees of correlation, from no correlation to weak correlation, between these 2 readings have been reported [12,15,16,18,19]. So it has been suggested that a direct estimation of heart iron overload using heart T2* imaging is more useful in evaluating the state of heart iron overload than relying on liver measurements [12].
As mentioned before, the liver inflammation like hepatitis, infections, and also liver damage might cause variation in serum ferritin levels [5]. The present study was performed to evaluate the relation between serum ferritin level, liver function tests, presence of hepatitis C antibody and the liver and heart iron concentrations assessed by means of MRI T2*, to evaluate if hepatitis C infection and liver function tests status can modify the relation between serum ferritin and liver and heart MRI T2* readings. We also aimed to assess the relation between liver and heart iron concentrations assessed by means of MRI T2* among our patients.
Methods
Patients
This was a cross-sectional study of regularly transfused thalassaemia major patients from Zafar Adult Thalassemia Center, a referral thalassaemia centre in Tehran, Islamic Republic of Iran, and was approved by the ethics committee of Shahid Beheshti University of Medical Sciences, Tehran. The study was conducted from May 2010 to September 2010.
The inclusion criteria were: being a major thalassaemia patient, regularly transfused, age over 15 years and receiving chelation therapy. Exclusion criteria were having any cardiac anomaly, clinical heart failure, and chelation therapy with a method other than using desferrioxamine. Data were obtained from the medical records of the patients and written consent was received from all patients whose records were used. All patients were under chelation therapy with desferrioxamine (20–50 mg/kg daily).
Sample size
The primary outcome of interest was the relation between ferritin and liver MRI T2* relaxation times. For a power of 95% to detect a correlation as strong as 0.3 with a one-sided type I error of 0.05, a sample size of 138 cases was calculated. As we expected that about 15% of our patient records would be incomplete, 160 patient records were randomly chosen by computer generated randomized numbers from 734 eligible records and the data extracted; this resulted in 156 cases with complete data included in the study.
Cardiac and hepatic MRI T2* as well as liver function test (aspartate aminotransferase, alanine aminotransferase) results, serum ferritin levels and hepatitis C antibody were extracted from the patients’ files. All measurements of serum ferritin, aspartate aminotransferase, alanine aminotransferase, hepatitis C antibody, and heart and liver MRI T2* were conducted within a 6-month period for all patients.
The methods used to measure liver enzymes, serum ferritin levels and hepatitis C antibody and obtain the cardiac and hepatic MRI T2*, and the statistical analysis are described in our previous paper [17] and are briefly repeated here.
Serum ferritin, liver function tests and hepatitis C antibody measurements
Serum ferritin and hepatitis C antibody were measured by electrochemiluminescence (Elecsys 2010 Chemistry Analyzer, Roche Diagnostics, Basel, Switzerland). Aspartate aminotransferase and alanine aminotransferase were measured using COBAS INTEGRA® 400 plus analyser (Roche Diagnostics, Basel, Switzerland).
Magnetic resonance imaging protocol
Patients were scanned using a Symphony 1.5T Scanner (Siemens, Germany). A standard radio frequency (RF) body coil was used in all measurements. The Royal Brompton protocol [20], which is based on a single breath-hold multi-echo gradient-echo sequence, was used for T2* measurements. The liver T2* was determined by imaging a single trans-axial slice (10 mm) through the centre of the liver. For the measurement of myocardial T2*, scans were synchronized to the cardiac cycle using standard ECG gating. A single 10-mm-thick short-axis mid-ventricular slice, positioned halfway between the base and the apex of the left ventricle (LV), was performed.
T2* calculations
T2* values were evaluated using in-house software (Noor Medical Imaging Center, Tehran). A homogeneous region of interest (ROI) was outlined in the liver parenchyma. A homogeneous full-thickness ROI was chosen in the ventricular septum. The mean signal intensity of region was measured for each image and plotted against the echo time.
Statistical analysis
Summary data are presented as mean and standard deviation (SD) and frequency (percentage). Chi-squares and t-tests were used to evaluate the differences in qualitative and quantitative variables respectively. Spearman’s correlation was used to assess the correlation between variables. To demonstrate these correlations we used scatter plots with a regression line and smooth Loess line. Multiple regression analysis was used to study the confounding or modifying effect of paraclinical findings (HCV antibody, aspartate aminotransferase, alanine aminotransferase) on the relation between ferritin and liver and cardiac T2*. All statistical analysis was performed with SPSS, version 17.0.
Results
One hundred and fifty six patients (84 male and 72 female) were evaluated in this study. Their mean age was 24.1 (SD 5.4) years and the mean transfusion duration was 22.3 (SD 5.1) years. Splenectomy had been performed in 77 (49.1%) of the patients. All patients had received iron chelation therapy from early childhood [mean therapy duration 20 (SD 7.5) years]. Patients’ demographic characteristics are shown in Table 1.
Paraclinical findings (HCV antibody, aspartate aminotransferase and alanine aminotransferase) of thalassaemia patients are presented in Table 2. Forty two patients (26.4%) were positive for HCV antibody. The results of cardiac and liver MRI T2* and serum ferritin levels in thalassaemia patients are also illustrated in Table 2.
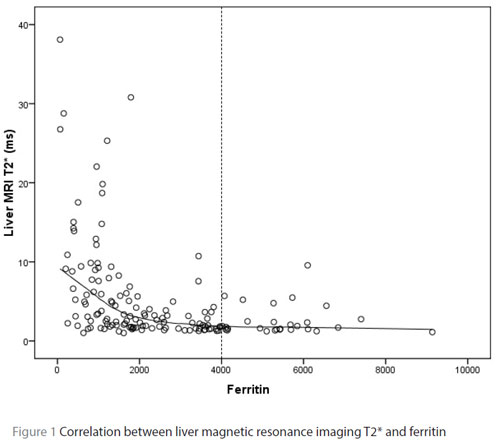
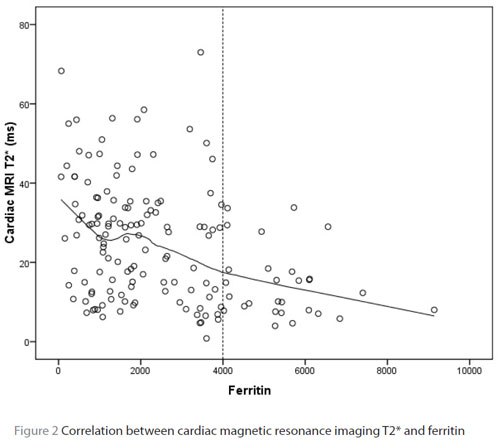
The correlation between serum ferritin levels, hepatic and cardiac MRI T2* is shown in Table 3. There was a moderate negative correlation between liver MRI T2* relaxation time and serum ferritin (r = –0.535, P < 0.001) (Table 3, Figure 1). Heart MRI T2* relaxation time was poorly correlated with serum ferritin (r = –0.361, P < 0.001) (Table 3, Figure 2). As is demonstrated in Table 3 and Figure 1, the correlation between serum ferritin and the liver T2* relaxation time greatly weakened in patients with ferritin readings higher than 4000 ng/mL.
A comparison of T2*status in patients with positive and negative HCV antibody is shown in Table 4. We did not find a statistically significant relation between liver MRI T2* and HCV antibody status of patients (P = 0.136). Liver MRI T2* was significantly lower in patients who had increased aspartate aminotransferase (P < 0.001) and alanine aminotransferase (P < 0.001) (Table 4). However multiple regression analysis revealed that HCV antibody, aspartate aminotransferase or alanine aminotransferase did not confound or modify the relation between ferritin and liver T2*, or that between ferritin and cardiac T2*(minimum P = 0.147).
There was a poor correlation between the liver and heart MRI T2* readings of the patients (r = 0. 281, P < 0.001).
Discussion
Our results indicate a moderate negative correlation between liver MRI T2* relaxation times and serum ferritin. This is in line with other studies estimating liver iron overload using MRI T2* [21–25]. However, an important aspect of our findings was the much weaker correlation between liver MRI T2* relaxation times and ferritin readings in higher concentrations of serum ferritin (> 4000 ng/mL). This indicates that serum ferritin levels cannot predict the liver iron content among this group of patients. This is in line with the findings Worwood et al. who reported that a simple relationship between serum ferritin and iron stores cannot be assumed when ferritin concentrations exceed 4000 ng/mL [26]. They suggested that the secretion of glycosylated ferritin from reticuloendothelial cells reaches a maximum with increasing iron accumulation, reflecting a maximum rate of synthesis.
We found poor correlation between heart MRI T2* relaxation times and serum ferritin indicating that serum ferritin cannot estimate the heart iron content. This finding further emphasizes the importance of MRI T2* as a more accurate method for estimating cardiac iron overload. Other studies have found different correlation strengths, ranging from no correlation to low correlation, between serum ferritin and heart iron content measured using MRI techniques, but all have found that serum ferritin cannot satisfactorily estimate cardiac haemosiderosis [11,12,15,27–29].
Our results also indicate that adding the effect of HCV antibody, aspartate aminotransferase and alanine aminotransferase in the model did not confound or modify the relation between ferritin and liver T2*, nor ferritin and cardiac T2*. This is in line with previous observations indicating that the MRI T2* liver iron accumulation index is not induced by the presence of chronic hepatitis C [30,31].
There was a poor correlation between the liver and heart MRI T2* readings among patients which concurs with other studies [12,15,16,18,19,32]. This emphasizes the important role of cardiac MRI T2* in evaluating heart iron content among thalassaemia patients instead of relying solely on liver MRI T2* readings to predict the heart iron content.
Our study suffers from some limitations namely, the use of HCV antibody to diagnose HCV positivity as this has a high rate of false positives compared to polymerase chain reaction. This might make our finding that the hepatitis C infection does not confound or modify the relationship between ferritin and liver or cardiac T2* unreliable to some degree. Moreover, the 6 months window in our study between measurements of hepatitis C antibody and MRI T2* could challenge the validity of the findings in a heavily transfused patient since in heavily transfused patients there is a chance of acquiring the infection in this time frame.
Conclusions
The serum ferritin level has a moderate predictive value for estimating the level of liver iron overload, but a poor predictive value for heart iron overload among thalassaemia major patients. Hepatitis C infection and raised liver function tests do not confound or modify the relation between ferritin and liver or cardiac T2*. The poor correlation between liver and heart MRI T2* suggests a need for direct evaluation of heart iron content. To better evaluate the haemosiderosis status among thalassaemia patients, a routine evaluation of liver and heart iron content using MRI T2* is suggested, if possible, instead of relying solely on serum ferritin.
Competing interests: None declared.
References
- Yesilipek MA. Stem cell transplantation in hemoglobinopathies. Hemoglobin, 2007, 31:251–256.
- Hamed EA, El Melegy NT. Renal functions in pediatric patients with beta-thalassemia major: relation to chelation therapy: original prospective study. Italian Journal of Pediatrics, 2010, 36:39.
- Economou M et al. Renal dysfunction in patients with beta-thalassemia major receiving iron chelation therapy either with deferoxamine and deferiprone or with deferasirox. Acta Haematologica, 2010, 123:148–152.
- Schrier SL, Angelucci E. New strategies in the treatment of the thalassemias. Annual Review of Medicine, 2005, 56:157–171.
- Cohen AR et al. Thalassemia. Hematology (American Society of Hematology Education Program). Washington DC, American Society of Hematology, 2004:14–34.
- Argyropoulou MI, Astrakas L. MRI evaluation of tissue iron burden in patients with beta-thalassaemia major. Pediatric Radiology, 2007, 37:1191–1200, quiz 1308–1309
- Wood JC. Magnetic resonance imaging measurement of iron overload. Current Opinion in Hematology, 2007, 14:183–190.
- St Pierre TG et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood, 2005, 105:855–861.
- Kirk P et al. International reproducibility of single breathhold T2* MR for cardiac and liver iron assessment among five thalassemia centers. Journal of Magnetic Resonance Imaging, 2010, 32:315–319.
- Tanner MA et al.; Thalassemia International Federation Heart T2* Investigators. Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica, 2006, 91:1388–1391.
- Kirk P et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation, 2009, 120:1961–1968.
- Anderson LJ et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European Heart Journal, 2001, 22:2171–2179.
- Anderson LJ. Assessment of iron overload with T2* magnetic resonance imaging. Progress in Cardiovascular Diseases, 2011, 54:287–294.
- Aessopos A, Berdoukas V, Tsironi M. The heart in transfusion dependent homozygous thalassaemia today–prediction, prevention and management. European Journal of Haematology, 2008, 80:93–106.
- Roghi A et al. Absence of cardiac siderosis despite hepatic iron overload in Italian patients with thalassemia intermedia: an MRI T2* study. Annals of Hematology, 2010, 89:585–589.
- Anderson LJ et al. Development of thalassaemic iron overload cardiomyopathy despite low liver iron levels and meticulous compliance to desferrioxamine. Acta Haematologica, 2006, 115:106–108.
- Hashemieh M et al. T2-star (T2*) magnetic resonance imaging for assessment of kidney iron overload in thalassemic patients. Archives of Iranian Medicine, 2012, 15:91–94.
- Deborah Chirnomas S et al. Practical implications of liver and heart iron load assessment by T2*-MRI in children and adults with transfusion-dependent anemias. American Journal of Hematology, 2008, 83:781–783.
- Tziomalos K, Perifanis V. Liver iron content determination by magnetic resonance imaging. World Journal of Gastroenterology, 2010, 16:1587–1597.
- Westwood M et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. Journal of Magnetic Resonance Imaging, 2003, 18(1):33–39.
- Ooi GC et al. Magnetic resonance screening of iron status in transfusion-dependent beta-thalassaemia patients. British Journal of Haematology, 2004, 124:385–390.
- Voskaridou E et al. Early markers of renal dysfunction in patients with sickle cell/beta-thalassemia. Kidney International, 2006, 69:2037–2042.
- Perifanis V et al. comparison of effects of different long-term iron-chelation regimens on myocardial and hepatic iron concentrations assessed with T2* magnetic resonance imaging in patients with beta-thalassemia major. International Journal of Hematology, 2007, 86:385–389.
- Mazza P et al. Iron overload in thalassemia: comparative analysis of magnetic resonance imaging, serum ferritin and iron content of the liver. Haematologica, 1995, 80:398–404.
- Zamani F et al. T2* magnetic resonance imaging of the liver in thalassemic patients in Iran. World Journal of Gastroenterology, 2011, 17:522–525.
- Worwood M et al. Binding of serum ferritin to concanavalin A: patients with homozygous beta thalassaemia and transfusional iron overload. British Journal of Haematology, 1980, 46:409–416.
- Leung AW et al. Magnetic resonance imaging assessment of cardiac and liver iron load in transfusion dependent patients. Pediatric Blood & Cancer, 2009, 53:1054–1059.
- Di Tucci AA et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica, 2008, 93:1385–1388.
- Fragasso A et al. Myocardial iron overload assessed by magnetic resonance imaging (MRI)T2* in multi-transfused patients with thalassemia and acquired anemias. European Journal of Internal Medicine, 2011, 22:62–65.
- Koliakos G et al. Urine biochemical markers of early renal dysfunction are associated with iron overload in beta-thalassaemia. Clinical and Laboratory Haematology, 2003, 25:105–109.
- Papakonstantinou O et al. Quantification of liver iron overload by T2 quantitative magnetic resonance imaging in thalassemia: impact of chronic hepatitis C on measurements. Journal of Pediatric Hematology/Oncology, 1999, 21:142–148
- Christoforidis A et al. Correlative study of iron accumulation in liver, myocardium, and pituitary assessed with MRI in young thalassemic patients. Journal of Pediatric Hematology/Oncology, 2006, 28:311–315.








