M.M. K. Boujan1 and K.H.S.H. Sharef2
1School of Medicine, University of Sulaimani, Sulaimani, Iraq (Correspondence to M.M. K. Boujan:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
2Paediatric Hospital, Sulaimani, Iraq.
Received: 08/02/12; accepted: 21/03/12
EMHJ, 2013, 19(5):502-505
Introduction
In 1894, Townsend described a self-limited bleeding condition that usually occurs 1–5 days after birth in patients with non-classic haemophilia [1]. The term hemorrhagic disease of the newborn was adopted to describe bleeding disorders among neonates associated with a traumatic birth or haemophilia [2]. This diagnostic term has now been replaced with the term vitamin K deficiency bleeding (VKDB) because vitamin K deficiency is not the sole cause of hemorrhagic disorders in preterm and term infants [3]. Vitamin K represents a group of lipophilic and hydrophobic vitamins that promotes the hepatic synthesis of the certain clotting factors [4,5].
Although some controversy surrounds postnatal timing of the initial haemorrhage, VKDB of the newborn is usually classified by 3 distinct time periods after birth: early-onset VKDB usually occurs during first 24 hours after birth and is very rare; classic VKDB usually occurs after 24 hours and as late as the first week of life and is also rare; and late-onset VKDB in a newborn who did not receive a vitamin K shot, and in those of Asian descent, which usually occurs between age 2–12 weeks but can be seen as long as 6 months after birth. The characteristics of VKDB are that it is most common in breastfed infants who did not receive vitamin K prophylaxis at birth. Vitamin K content is low in mature human milk and ranges from 1–4 µg/L. Industrial contaminants in breastmilk have been implicated in promoting VKDB. More than half of these infants present with acute intracranial haemorrhages [6]. In addition, infants who have intestinal malabsorption defects (cholestatic jaundice, cystic fibrosis, etc.) may also have late VKDB.
The rate of late-onset VKDB, often manifesting as sudden central nervous system haemorrhage, ranges from 4.4 to 7.2 per 100 000 births, according to reports from Europe and Asia. Normally, VKDB infants do not require surgical care but in rare cases, an infant may need neurosurgical evaluation and treatment [7,8]. Other conditions, such as those associated with short-bowel syndrome and hepatobiliary disease may require surgical evaluation. Intracranial bleeding is rare and is usually associated with other causes of bleeding, particularly thrombocytopenia; however, intracranial haemorrhage has been reported in VKDB and can be fatal [9].
We present here a case of spontaneous subdural haematoma in a previously healthy 40-day-old infant and discuss the subsequent changes in our hospital protocol regarding the use of vitamin K prophylaxis in newborns.
Case report
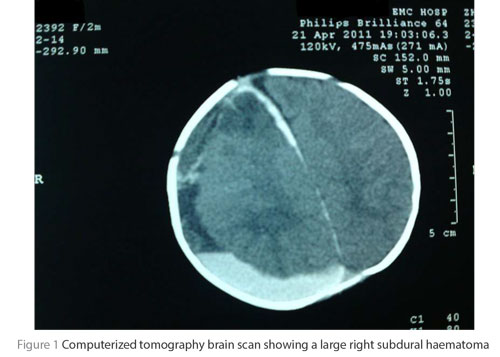
A 40-day-old male infant presented to the casualty department of our hospital unconscious for 1 day duration. His Glasgow coma scale was 4 (E1 V1 M2), with no eye opening to stimulation, no crying to stimulation and movement only to painful stimulation and with left-sided hemiparesis. The patient was intubated and admitted to the intensive care unit. His computerized tomography (CT) brain scan showed a large right subdural haematoma (Figure 1). No history or signs of trauma were observed. No blood disease history was reported by the family.
The child had had an uneventful antenatal care period. The mother had given birth in the hospital with a normal vaginal delivery of a full-term newborn weighing 3 kg. The child did not receive vitamin K injection. The child was breastfed. His mother gave no history of ingestion of any medications during pregnancy. Parents gave a history of an uncomplicated circumcision at the age of 14 days.
The child’s blood investigation revealed the followings: prothrombin time (PT) 35.0 s (control 14.0 s), international normalized ratio (INR) 3.7, activated partial thromboplastin time (aPTT) 35.0 s (control 32.0 s), haemoglobin 5.2 g/dL. Blood film showed that red blood cells were normochromic with anisocytosis, few spherocytosis cells, blister cells and slight polychromasia; white blood cells showed no primitive cells; and platelets count was 563 ×1000 /mm3 (normal 150–500 × 1000). The conclusion from the blood film analysis was severe anaemia and that vitamin K deficiency should be excluded.
For the first and second day of admission fresh-frozen plasma was infused, and vitamin K injections (10 mg once daily) and factor 7 were given. Within 2 days the child’s haematology was as follows: INR had dropped to 1.04 (normal), PT was 14.9 s, (control 14.2 s) and aPTT was 31.3 se.
The child was prepared for craniotomy and a haematoma was evacuated. Postoperative CT showed resolution of most of the haematoma. The child regained full consciousness 5 days after the operation; muscle power of the left side was back to normal. There were no postoperative complications.
On follow-up at 9 months, the child was well and had normal milestones.
Discussion
Newborn infants are at risk for vitamin K deficiency because their immature liver does not efficiently utilize vitamin K. In addition, they tend to have low vitamin K stores because of the low vitamin K content of breastmilk, a sterile gut and poor placental transfer of vitamin K. The risk of developing VKDB is further increased by maternal ingestion of coumarin, certain antibiotics (i.e. cephalosporins) and some anticonvulsants during pregnancy [10].
On 13 July 2006, before this current patient, we received another child of the same age with the similar condition, although the child was saved by a craniotomy and haematoma evacuation, nothing was done to address this problem at that time.
Since 1961, awareness has been raised about the role of routine administration of vitamin K to newborn babies and its role in preventing the rather uncommon condition of late-onset VKBD.
The following questions concerning administration of vitamin K to newborns were raised:
- Should it be given routinely to every newborn or just at-risk babies?
- Is there a relationship between vitamin K and child oncology?
- Which is the more effective route of administration: parenteral or oral?
- Which treatment is more cost-effective?
- What if parents refuse vitamin K prophylaxis?
The efficacy of neonatal vitamin K prophylaxis (oral or parenteral) in the prevention of early VKDB is firmly established. It has been the standard of care since the American Academy of Pediatrics (AAP) recommended it in 1961 [11]. A single dose of oral vitamin K has been used for neonatal prophylaxis, the rate has decreased to 1.4 to 6.4 per 100 000 births. Parenteral neonatal vitamin K prophylaxis prevents the development of late-onset VKDB in infants, with the rare exception of those with severe malabsorption syndromes [7,8]. However, parents’ refusal of prophylaxis and an increasing frequency of breastfeeding may be causing a resurgence of VKDB in developed countries [5].
The AAP Committee on Nutrition, stated in its report recommendations and conclusions that vitamin K1 is to be administered for the preterm as well as full-term newborns as a prophylactic treatment against spontaneous haemorrhage. The recommended dose was 0.5–1.0 mg parenteral or 1.0–2.0 mg oral, with larger doses for children of mothers on anticoagulants. It also stated that it is ineffective to administer vitamin K to the mother herself [11].
The APP Committee on the Fetus and Newborn restudied the subject in 2002–03 and published its policy statement that vitamin K1 should be given to all newborns as a single, intramuscular dose of 0.5–1 mg. They also recommended that additional research should be conducted on the efficacy, safety, and bioavailability of oral formulations and optimal dosing regimens of vitamin K to prevent late VKDB. The committee also advised that health care professionals should promote awareness among families of the risks of late VKDB associated with inadequate vitamin K prophylaxis from current oral dosage regimens, particularly for newborns who are breastfed exclusively [12–14].
Other countries developed their own policies regarding recommending vitamin K administration for newborn children, after extensive studies. Canada, for instance, through the Fetus and Newborn Committee of the Canadian Pediatric Society, and the Committee on Child and Adolescent Health, College of Family Physicians of Canada, reaffirmed in 2011 that vitamin K1 should be given as a single intramuscular dose of 0.5 mg (birth weight ≤ 1500 g or less) or 1.0 mg (birth weight > 1500 g) to all newborns within the first 6 h after birth. For newborn infants whose parents refuse an intramuscular injection, an oral dose of 2.0 mg vitamin K1 can be given at the time of the first feeding. This should be repeated at 2–4 weeks and 6–8 weeks of age [15].
In New Zealand the 2000 consensus statement recommended the intramuscular route of administration for vitamin K given at birth (for term babies 0.5–1 mg soon after birth; for preterm babies 0.5 mg soon after birth). If parents do not consent to intramuscular injection but consent to oral vitamin K, this needs to be given according to the following regime: 2 mg oral soon after birth, 2 mg oral at 3–7 days, 2 mg oral at 6 weeks [16].
In Australia, the National Health and Medical Research Council joint statement and recommendations on Vitamin K administration to newborn infants to prevent VKDB in infancy in 2010 was that all newborn infants should receive vitamin K prophylaxis and that healthy newborn infants should receive vitamin K either: by intramuscular injection of 1 mg (0.1 mL) of Konakion® MMI or 3× 2 mg (0.2 mL) oral doses of Konakion® MM, given at birth; or at the time of newborn screening (usually at 3–5 days of age) and in the 4th week [17].
In the United Kingdom, the National Institute for Health and Clinical Excellence, guideline CG37 (postnatal care and vitamin K) was that all parents should be offered vitamin K prophylaxis for their babies to prevent the rare but serious and sometimes fatal disorder of VKDB. Vitamin K should be administered as a single dose of 1 mg intramuscular as this is the most clinically and cost-effective method of administration. If parents decline intramuscular vitamin K for their baby, oral vitamin K should be offered as a second-line option. Parents should be advised that oral vitamin K must be given according to the manufacturer’s instructions for clinical efficacy and will require multiple doses [18].
A large national study, the United Kingdom Childhood Cancer Study, included an updated pooled analysis with data for 7017 children (1174 with leukaemia). It found no association between intramuscular vitamin K and any diagnostic group. The authors concluded that in the light of all available evidence, chance was the most likely explanation for early findings regarding the link between vitamin K and childhood cancer [19]. These guidelines for management of child with this type of bleeding were to immediately administer vitamin K subcutaneously (hold pressure on the site) for any infant in whom VKDB is suspected or who has serious, unexplained neonatal bleeding. Fresh-frozen plasma may be considered for moderate-to-severe bleeding [20]. In case of intracranial haemorrhage, evacuation of life-threatening haematoma should be considered [21].
Conclusion and recommendations
Our experience in Sulaimani is that prior to the case reported here only at-risk babies (e.g. premature babies) were given 1 mg vitamin K in the form of a single injection of vitamin K1 to the thigh. Following this patient actions taken in our hospital are now:
- routine injection of newborns with vitamin K;
- reporting spontaneous bleeding conditions in children; and
- increasing the awareness of health professionals for this condition.
We recommend expanding this protocol to other parts of Kurdistan and hospitals of Iraq. A wide prospective study on Iraqi babies and their parents should be done regarding the effect of this prophylactic regimen preventing future VKDB in our country. The following points should be addressed:
- parental consent to administration of vitamin K to their newborn children;
- circumstances when babies miss their prophylactic; and
- education for midwives about the seriousness and types of this VKDB, in case of house delivery.
- Consent: Written informed consent was obtained from the patient’s parents for publication of this case report and the accompanying image.
Competing interests: None declared
References
- Bandyopadhyay PK. Vitamin K dependent γ-glutamylcarboxylation: an ancient posttranslational modification. Vitamins and Hormones, Elsevier Inc, 2008, 78:157–184.
- Victora C. Vitamin K deficiency and haemorrhagic disease of the newborn. A public health problem in less developed countries? New York, United Nations Children’s Fund, 1997 (UNICEF Staff Working Papers, Evaluation, Policy and Planning Series No. EVL-97-005).
- Sutor AH et al; International Society on Thrombosis and Haemostasis. Vitamin K deficiency bleeding (VKBD) in infancy. ISTH Pediatric/Perinatal Subcommittees. Thrombosis and Haemostasis, 1999, 81:456–461.
- Oldenburg J et al. The vitamin K cycle. Vitamins and Hormones, 2008, 78:35–62.
- McNinch A, Busfield A, Tripp J. Vitamin K deficiency bleeding in Great Britain and Ireland: British Paediatric Surveillance Unit Surveys, 1993–94 and 2001–02. Archives of Disease in Childhood, 2007, 92:759–766.
- Pichler E, Pichler L. The neonatal coagulation system and the vitamin K deficiency bleeding—a mini review. [Das neonatale Gerinnungssystem und die Vitamin K Mangelblutung—eine kurze Übersicht.] Wiener Medizinische Wochenschrift, 2008, 158:385–395.
- Von Kries R, Hanawa Y. Report of Scientific and Standardization Subcommittee on Perinatal Haemostasis. Neonatal vitamin K prophylaxis. Thrombosis and Haemostasis, 1993, 69:293–295.
- Motohara K, Endo F, Matsuda I. Screening for late neonatal vitamin K deficiency by acarboxyprothrombin in dried blood spots. Archives of Disease in Childhood, 1987, 62:370–375.
- Greer FR et al. Improving the vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics, 1997, 99:88–92.
- Olson R. Vitamin K. In: Shils M et al., eds. Modern nutrition in health and disease. Philadelphia, Lippincott, 2000:363.
- American Academy of Pediatrics, Committee on Nutrition. Vitamin K compounds and the water-soluble analogues: use in therapy and prophylaxis in pediatrics. Pediatrics, 1961, 28:501–507.
- American Academy of Pediatrics Committee on Fetus and Newborn. Controversies concerning vitamin K and the newborn. Pediatrics, 2003, 112:191–192.
- American Academy of Pediatrics. AAP publications reaffirmed, May 2006. Pediatrics, 2003, 112:191–192.
- Policy statement—AAP publications retired and reaffirmed. Pediatrics, 2009, 124:845.
- McMillan D; Canadian Paediatric Society, Fetus and Newborn Committee. Position statement. Routine administration of vitamin K to newborns. Pediatric Child Health, 1997, 2(6):429–431.
- Vitamin K. NZCOM Consensus Statement. New Zeeland College of Midwives [online] (http://www.midwife.org.nz/index.cfm/3,108,559/vitamin-k-2000.pdf, accessed 26 February 2013).
- Joint statement and recommendations on vitamin K administration to newborn infants to prevent vitamin K deficiency bleeding in infancy. Canberra, National Health and Medical Research Council, 2010 (NHMRC publication reference CH54).
- Postnatal care: routine postnatal care of women and their babies. NICE guideline CG37. London, United Kingdom, National Institute for Health and Clinical Excellence, 2006.
- Fear NT et al.; United Kingdom Childhood Cancer Study. Vitamin K and childhood cancer: a report from the United Kingdom Childhood Cancer Study. British Journal of Cancer, 2003, 89:1228–1231.
- Greer FR et al. Improving the vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics, 1997, 99:88–92.
- Narayan RK, Kempisty S. Closed head injury. Principles of Neurosurgery, 2005, 19:301–317.




 Volume 31, number 5 May 2025
Volume 31, number 5 May 2025 WHO Bulletin
WHO Bulletin Pan American Journal of Public Health
Pan American Journal of Public Health The WHO South-East Asia Journal of Public Health (WHO SEAJPH)
The WHO South-East Asia Journal of Public Health (WHO SEAJPH)