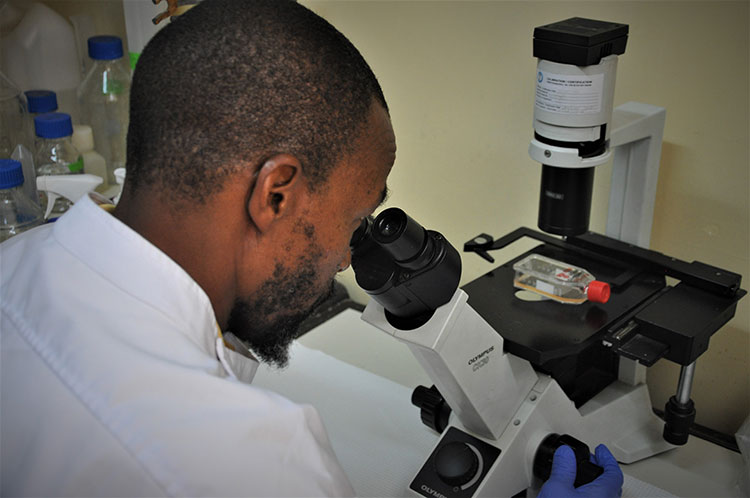
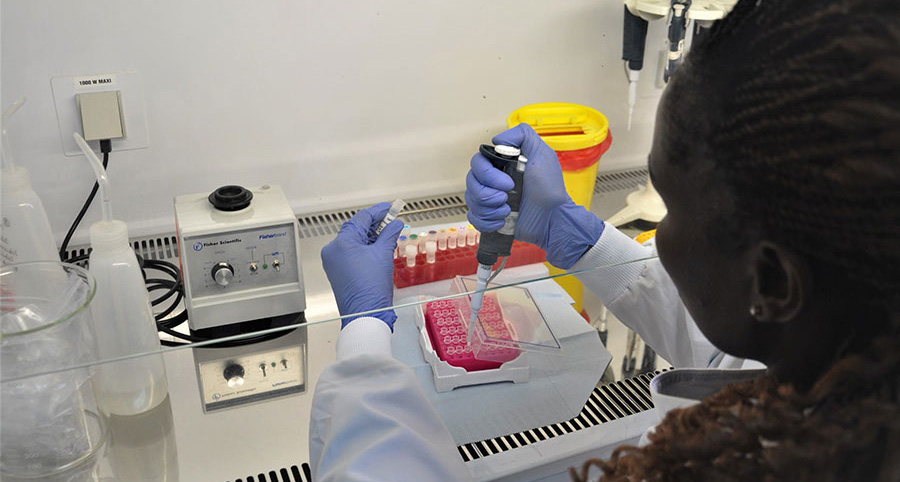
 Laboratory technologist Agnes Chepkurui prepares samples for the final stage of testing for polioviruses within the laboratory. This stage of the process is conducted if suspected polioviruses are identified in the cell culture under a microscope, in order to find out what kind of virus it is. WHO/L.Dore
Laboratory technologist Agnes Chepkurui prepares samples for the final stage of testing for polioviruses within the laboratory. This stage of the process is conducted if suspected polioviruses are identified in the cell culture under a microscope, in order to find out what kind of virus it is. WHO/L.Dore
Although it refers to events that will happen after the end of poliovirus transmission, containment is a vital step on the road to eradication. It includes safeguards, biosafety and biosecurity requirements to minimize the risk that poliovirus might be released from laboratories, vaccine production facilities or other facilities that handle or store eradicated poliovirus.
What happens after eradication
After eradication, polioviruses will be kept in a limited number of laboratories and vaccine manufacturing facilities worldwide. Effective containment of these viruses includes safeguards to minimize the risk of their release into communities, which could have devastating consequences. The WHO Global Action Plan III provides guidance for countries to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of OPV use.
How polio containment works
Two of the three types of poliovirus have already been declared eradicated by the Global Commission for the Certification of Eradication of Poliomyelitis (GCC): wild poliovirus type 2 in September 2015, and wild poliovirus type 3 in October 2019. Wild poliovirus type 1 is the only strain that remains in circulation.
After each type of poliovirus is eradicated, countries are strongly encouraged to destroy their poliovirus materials or transfer them to a government designated poliovirus-essential facility (PEF). Any country opting to retain materials for reasons such as vaccine production and critical research, must undergo certification of their designated poliovirus-essential facilities in agreement with the GAPIII Containment Certification Scheme (GAPIII-CCS).
Ensuring strict biosafety and biosecurity requirements are met in every facility that handles or stores eradicated polioviruses minimizes the risk of these viruses being released into the community. Limiting the number of facilities holding the virus, to a minimum, greatly reduces risk and strengthens the likelihood that global containment standards can be met and maintained.
Containment in the Eastern Mediterranean Region
At the 71st World Health Assembly in May 2018, Member States adopted a resolution on containment urging the intensification of efforts to accelerate progress towards poliovirus containment globally.
Two EMR countries, Iran and Pakistan, have opted to become polio-essential facilities. Iran is planning to host two polio-essential facilities, the RAZI Institute and Institute Pasteur, to retain poliovirus vaccine production and quality control, while the Pakistan polio-essential facility may retain poliovirus strains for polio serological testing.
Following the certification of eradication of WPV3 in October 2019, the focus is on ensuring countries conduct inventories and destroy wild and VDPV type 3 materials and/or designate facilities for their retention if critical.
Activities are underway to ensure that all poliovirus type 2 materials are destroyed, or safely and securely contained. Countries have conducted national inventories of facilities that handle or store wild and vaccine-derived poliovirus type 2, and have transferred to PEFs or destroyed unneeded virus. Similar activities for facilities that handle or store OPV2 and Sabin 2 materials are in progress.
Working towards certification
Certification of polio eradication is conducted on a regional basis. Each region can consider certification only when all countries in the area demonstrate the absence of wild poliovirus transmission for at least three consecutive years in the presence of certification standard surveillance. Twenty out of the 22 countries in the EMR have been certified as wild poliovirus-free. The remaining two are Afghanistan and Pakistan, where wild poliovirus type 1 still circulates. Wild poliovirus types 2 and 3 have been globally certified.
The Eastern Mediterranean Regional Certification Commission (EM/RCC) was established in 1995 and has been meeting annually and regularly since then. Thirty-five meetings have been conducted, with the latest conducted virtually from 1-3 June 2021.
The EM/RCC tracks the progress of Member States and decides on certification status depending on the ability of the country to demonstrate the absence of wild poliovirus transmission for at least three consecutive years in the presence of certification standard surveillance and the annual ability to maintain this. Note that the emergence of vaccine-derived poliovirus is not yet considered a component for certification.
All countries except the two endemic countries have submitted their basic and final national documentation for certification, and documentation has been accepted in all cases. The list of certified countries includes Bahrain, Djibouti, Egypt, Islamic Republic of Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Occupied Palestinian Territory, Qatar, Saudi Arabia, Somalia, Sudan, Syrian Arab Republic, Tunisia, United Arab Emirates and Yemen.
More information on progress towards regional certification
Learn more about the Regional Certification Commission and its members
Read the Eastern Mediterranean Regional Certification Commission intercountry meeting reports
Review information on individual country certification
Intercountry meetings for directors of poliovirus laboratories