Brussels Conference VII
Cholera situation reports
Cholera situation report, 23 November 2023
Cholera situation report, 23 October 2023
Cholera situation report, 20 September 2023
Cholera situation report, 18 July 2023
Cholera situation report, 17 June 2023
Cholera situation report, 8 April 2023
Cholera situation report, 3 April 2023
Cholera situation report, 20 March 2023
Cholera situation report, 28 February 2023
Cholera situation report, 31 January 2023
Cholera situation report, 15 January 2023
Cholera situation report, 18 December 2022
Cholera situation report, 10 December 2022
Cholera situation report, 29 November 2022
Cholera situation report, 14 November 2022
Cholera situation report, 7 November 2022
Cholera situation report, 1 November 2022
Cholera situation report, 23 October 2022
Cholera situation report, 14 October 2022
 Samples are being collected from different water sources (main network, wells, tanks, ice factories), and environment samples from the sewage system, Euphrates River. All samples are tested at Central Public Health Laboratory.
Samples are being collected from different water sources (main network, wells, tanks, ice factories), and environment samples from the sewage system, Euphrates River. All samples are tested at Central Public Health Laboratory.
Opening remarks by Dr Akjemal Magtymova, WHO Representative in Syria

Your Excellency, Minister of Health, Dr Hasan Al Gabbash;
Distinguished representatives of the Ministries of Education, Higher Education, Social Affairs and Labour, Information, and Interior;
Representatives of professional associations and syndicates;
UN sister agencies and international organizations; dear colleagues.
Today I am representing the WHO, but once upon a time I was an Ob/Gyn, having chosen that path after another woman, whom I admire till this moment: a professional who had passionately dedicated her life to saving lives of girls, women, precious lives of mothers, and their new-born children. I joined UNFPA after some years of clinical practice as engaging at strategic and population level meant one could save more lives at a given time through the right policies, strategies and plans to support interventions at different levels.
International community’s actions then have been guided by the Millennium Development Goals and the International Conference on Population and Development, held in Cairo in 1994, where diverse views on human rights, population, SRH, gender equality and sustainable development merged into a remarkable global consensus that placed individual dignity and human rights as well as reproductive rights at the very centre of development. A more recent population conference was held in Nairobi 2021, that called for accelerated action for justice and development.
We have had successes to celebrate for the past decades. Between 2000-2019, maternal mortality has fallen by 38%. Today, more women give birth with the help of a skilled health worker. Annual infant deaths have declined from 8.7 million in 1990 to 4.0 million in 2018. The global under-5 mortality rate has halved.
But as you all know, we still face many significant challenges:
Every day, 810 women still die from preventable cases related to pregnancy and childbirth.
Cervical cancer affects over half a million women each year killing a quarter of a million.
Every day, there are more than one million new cases of curable STIs and those which are growing resistant to antimicrobials.
An estimated 1 in 5 girls, or 12 million a year, are married before their 18th birthday, undermining their health, education, safety and opportunities.
And 1 in 3 girls and women will experience physical or sexual violence during their lifetime.
These are only some of the challenges.
Predictably, the poorest, and most vulnerable groups are over-represented in these numbers. Women, girls and children in fragile, conflict and violence-affected situations are especially vulnerable.
The common factor that could improve the chances of health, and indeed survival, for millions is universal health coverage, access to essential health services, the targets set forth in the agenda of SDGs. The Global Action Plan on Health and Well-Being for All, launched at the UN General Assembly in September, brings together 12 multilateral agencies, including UNFPA, WHO, UNICEF represented here, to support countries in accelerating progress towards the SDG health targets.
Countries, including Syria, also align their national policies with the Sustainable Development Goals by committing to improving the health of women, children, and adolescents through multi-sectoral and multi-partnership interventions, reducing gender and equity gaps, and improving the quality of services.
Many things have been achieved to address the reproductive, maternal and child health in Syria. But, for all our progress, the milestones of which I spoke are a distant dream for millions of mothers and their children. And this is the source of discontent. The ongoing 11-year crisis has profoundly impacted the health of women and children; too many are still dying because of lack of access to quality health care, resources, and information. I must also mention declining socio-economic situation that impacts health through indirect determinants, power to give warmth, clean water, sanitation, access to diagnostics and treatment, out-of-pocket expenditures.
I would like to commend the collective efforts and commitment for formulating the strategy to address these ongoing challenges. The adoption of the National Strategy on Reproductive, Maternal, Neonatal, Child and Adolescent Health for 2022 – 2025 developed by the Ministry of Health in close partnership with WHO, UNICEF and UNFPA was guided by the Global Strategy on Women's and Children's Health for 2016-2030 that focuses on survival, growth, and transformation. The National Strategy breathes new life and is one important milestone in achieving health for mothers & children; & achieving health for all.
As WHO, we have been there from the first day of the Strategy conceptualization, situation analysis and development and we stand committed in its steady implementation through operational planning with indicators’ framework, and costing. We have been supporting the reproductive, maternal, and children’s health in many ways and determined to continue. These include some of the most recent work, such as:
Integrated Management of Childhood Illnesses programme through 540 health centres that received over 1 million child patient visits in a year.
Nutrition surveillance through 968 health centres that provided over 1 million services and detected 20 000 cases of malnutrition.
Nineteen (19) stabilization centres that treated over 1500 complicated cases of malnutrition.
Infant and young child feeding programme in 761 health centres, providing more than 285 000 services.
Neonatal resuscitation programme in 38 hospitals that offered different interventions to prevent asphyxia in more than 70 000 deliveries.
Youth-friendly centres that provided over 25 000 services.
Newborn care-at-home programme that conducted 20 000 home visits to detect risk signs in pregnant women and newborns and referred them, when needed, to special care.
Capacity of reproductive health professionals built through specialized training courses to 630 midwives.
Development and distribution of reprodcutive guidelines and awareness-raising materials on pre-marital testing, sexually transmitted infections and family planning.
WHO remains firmly committed to supporting Syria in implementation of the strategy, and we are hopeful that this strategy will contribute to ensuring all women’s, new-borns,’ children’s and adolescents’ equitable access to comprehensive, integrated, high-quality, effective, and sustainable health services in Syria. More will need to be done as we move forward, for operationalization of the strategy and setting indicators’ framework, costing and financing its implementation.
I know from my own experience that more can be achieved when we work together within the government sectors, national and international organizations, and the whole society - we all can contribute to the implementation of the strategy, so that mothers can experience safe childbirth, babies are born healthy and develop to their fullest potential from birth to their adolescence and adult life.
Once again, I congratulate the Ministry of Health for launching this milestone Strategy and wish the Ministry and other health partners success in its implementation.
External news
6 Sept 2021 A Syrian medical source warns that COVID cases will double this fall as the Delta variant is spreading
4 Sept 2021 UN humanitarian chief concludes trip to Syria, Lebanon, Turkey
4 Sept 2021 Syria: Prevent ‘entire generation from being lost’, urges UN humanitarian chief
4 Sept 2021 Delta variant cannot be tested in hospitals under Syrian Governement
3 Sept 2021 MOH approves of vaccinating those who are above 18 years of age
3 Sept 2021 WHO: we have not sent tests to detect the “Delta variant” to Syria
3 Sept 2021 GoS uses Corona Vaccines to pursue its opponents
1 Sept 2021 WHO inaugurates the Center for research and early detection of epidemics in Berlin
1 Sept 2021 MoH: the presence of the “Delta” variant has to been detected It has not been in Syria
30 Aug 2021 Syria’s Ministry of Education opens schools amid warning of Delta coronavirus variant
30 Aug 2021 As the school opening approaches, WHO recommends vaccinating teachers, as a priority group
30 Aug 2021 WHO recommends that teachers be treated as a priority group concerning vaccination
30 Aug 2021 WHO and UNICEF oppose any future school closures
29 Aug 2021 Syria ,UN discuss cooperation to overcome war damage and siege
29 Aug 2021 Damascus ‘Thwarts’ Settlement as it Eyes Reconstruction
29 Aug 2021 Mikdad, Griffiths discuss aspects of international humanitarian work in Syria
25 Aug 2021 MoH announces the number of vaccinated people in Syria
18 Aug 2021 The arrival of the second batch of “AstraZeneca” vaccine to NES
16 Aug 2021 Calls for imposing restrictions in Busra al-Sham (Daraa) after an increase in COVID cases
15 Aug 2021 Second dose against coronavirus vaccination starts in Syria’s northeast IDP camp
10 Aug 2021 A study to establish a center to identify COVID variants in Syria.
9 Aug 2021 An increase in the number of COVID cases in “Al-Mujtahid” Hospital in Damascus
9 Aug 2021 WHO condemns participation of ambulances in celebrations supporting the Assad regime
9 Aug 2021 Through Russian cooperation, a study to establish a sequencing lab for Corona variants in Syria
5 Aug 2021 Launch of "2nd dose" vaccination campaign in camps in NES
30 July 2021 Syria receives 150,000 doses of Chinese COVID-19 vaccines
27 July 2021 Syrian & Russian efforts to restore water to Al-Hassakeh
24 July 2021 In Syria, health services at public hospitals are gratis on paper only
20 July 2021 The Delta variant exists in Syria. Back and muscle pain are some of its symptoms
14 July 2021 What is the latest data about COVID19 in State-Controlled-Areas
14 July 2021 Rebel-held Syria braces for coronavirus 'tsunami' -- without soap, running water
9 July 2021 In pictures: The suffering of the people of Al-Hasakah increases with continued water interruptions
7 July 2021 WHO Head of Mission in Syria conducts a visit to Al-Qamishli
6 July 2021 WHO promotes for the Syrian Goverenment in northeast Syria
6 July 2021 Hasaka Governor, Magtymova discuss impact of cutting off water by Turkish regime
6 July 2021 WHO Representative in Syria visits Qamishli and Al-Hassakeh
6 July 2021 WHO: Fears of the spread of epidemics due to water outages in Al-Hasakah
6 July 2021 Al-Hasakah Governor urges the ICRC to pressure the Turkish occupier to neutralize the Allouk station…
5 July 2021 Governor of Al-Hasakah discusses with Magtymova the results of cutting off drinking water by the Turkish regime and its mercenaries
5 July 2021 WFP explains the strategies of the Syrians to survive
4 July 2021 Despite all, we have to do more to our people
1 July 2021 Syrians and foreigners, who will come to Syria, will be exempted from PCR document if they had a vaccination
1 July 2021 WHO recommendations to mitigate the spread of black fungus due to COVID19
30 June 2021 Washington excludes “Corona” equipment in Syria from sanction
29 June 2021 AANES is awaiting more vaccines
29 June 2021 WHO data reveals that the vaccination rate in Syria is only at 0.58% now
28 June 2021 The Syrian Ministry of Health conceals COVID19 vaccination rates in Syria
25 June 2021 AstraZeneca reaches Al-Hol camp
24 June 2021 Supported by WHO, an Information Session about Corona
24 June 2021 An increase in the suicide rates in areas under the Syrian Government control
24 June 2021 Over 200 vaccinated in Syria's Al-Hol camp
24 June 2021 Chinese envoy calls for removal of sanctions against Syria
23 June 2021 New conflict ignited by United Nations dealings with the Syrian regime
23 June 2021 IDPs in Rojava suffering amid medicine shortages
21 June 2021 Syria’s tycoons getting richer a year after Caesar Act enaction
17 June 2021 Fears of coronavirus vaccine in Damascus amid lack of preventative measures
15 June 2021 An X-Ray machine has been put into service at the Al-Hasakeh Medical Center
15 June 2021 Syria's Addition to WHO Board Despite Hospital Attacks Draws Outcry
15 June 2021 Vaccinating 100 people in Newroz and Roj Camps against Corona
15 June 2021 World Health Organization welcomes Syria to its Executive Board despite protests
14 June 2021 Joan Mustafa: Those who received a dose of the Corona virus vaccine did not show any symptoms
13 June 2021 Global Health denies the existence of details of the arrival of a second batch of Corona vaccine to Syria
13 June 2021 Magtymova to “Sputnik”: Vaccination of health personnel agains C-19 in NES has been completed
13 June 2021 COVID-19 Vaccinations Campaigns Held in Kurdish-Held Regions East of Euphrates
13 June 2021 WHO condemns attacks on hospital in Afrin, northwest Syria
12 June 2021 42 NGOs warn of obstructing aid access to Syria
11 June 2021 The WHO's Orwellian relationship with Syria
9 June 2021 The Health Committee in Raqqa continues to vaccinate the elderly and health staff with the Corona vaccine
8 June 2021 Vaccinating residents with coronavirus starts in Syria’s Raqqa
7 June 2021 WHO comments regarding Syria's membership in its executive board
4 June 2021 First participation of Syria in activities of WHO Executive Council
30 May 2021 WHO delivers the first batch of Corona vaccine to SDF-controlled areas
27 May 2021 WHA approves resolution on health conditions in occupied Palestinian territory, Syrian Golan
26 May 2021 WHO hands over Syria’s Autonomous Administration Covid-19 vaccine
26 May 2021 Syria aid at risk in Security Council vote
25 May 2021 Report: Investing in Mental Health is Critical to Rebuilding Syria
17 May 2021 From Turkmenistan and Italy, Two Women Blaze New Trails
12 May 2021 COVID-19 Vaccination Campaign in Damascus and Idlib
11 May 2021 Despite their military use, WHO delivers ambulances to the Assad Regime
11 May 2021 'It's all a lie': hesitancy hampers vaccine drive in war-scarred Syrian area
10 May 2021 Strengthening ambulance services as part of the public health emergency response in Syria
9 May 2021 Health Ministry receives 40 ambulances provided by WHO
9 May 2021 40 advanced ambulances from WHO
9 May 2021 MoH received 40 equipped ambulances from the World Health Organization
9 May 2021 Rojava has not received a single dose of COVID vaccine: health official
9 May 2021 AANES criticizes receiving only 645 doses from COVAX
7 May 2021 COVAX to buy two new COVID-19 vaccines
7 May 2021 MoH calls for online registration to receive COVID-19 vaccine
7 May 2021 AANES accuses WHO of unfair distribution of “Corona” vaccines
6 May 2021 Syria launches online registration for vaccination against COVID-19
6 May 2021 Syria: Hospitals run out of supplies as second COVID-19 wave hits
6 May 2021 COVID-19 vaccines will reach Syria. Who will get them?
6 May 2021 Northeastern Syria: Hospitals run out of funds and supplies as second COVID-19 wave hits the region
5 May 2021 Northeastern Syria: Hospitals run out of funds and supplies as second COVID-19 wave hits region
4 May 2021 ‘Do the right thing’ and protect civilian infrastructure during conflict: Lowcock
5 May 2021 Vaccine campaign begins amid virus surge in rebel-held Syria
3 May 2021 Vaccine rollout begins in rebel-held northern Syria
2 May 2021 Vaccination campaign begins in Northwest Syria
1 May 2021 COVID cases surge in north-eastern Syria amid oxygen shortages
1 May 2021 Northeast Syria ‘overwhelmed’ by COVID-19 spike, test shortage
1 May 2021 Chinese envoy calls for political settlement of Syria crisis
30 April 2021 Syria Government Receives First COVAX Vaccines
29 April 2021 Syria gets donation of 150,000 COVID shots from China.
25 April 2021 Syria receives batch of UN vaccines to speed up virus fight
24 April 2021 Syria receives more than 200,000 doses of anti-Covid-19 vaccines
23 April 2021 Syria receives first COVID-19 vaccines, for most vulnerable
23 April 2021 Syria receives 203,000 vaccine doses from UN to speed up inoculation process
22 April 2021 Syria receives its first delivery of COVID-19 vaccines through the COVAX
22 April 2021 Syria government receives first COVAX jabs
22 April 2021 Syria receives over 200,000 doses of AstraZeneca vaccine under UN-backed COVAX scheme
22 April 2021 WHO, GAVI alliance deliver 200,000 doses of AZ: UN
22 April 2021 'A great day of hope': War-torn Syria receives first Covid vaccines
22 April 2021 COVID-19: What you need to know about the coronavirus pandemic on 22 April
22 April 2021 Syria receives first batch of COVID-19 vaccines through COVAX Facility
22 April 2021 Syria receives its first delivery of COVID-19 vaccines through the COVAX Facility
22 April 2021 Health Ministry receives first batch of Covid-19 vaccines provided by COVAX
22 April 2021 Syria Receives 203,000 COVID-19 Vaccines
22 April 2021 Syria receives batch of UN vaccines to speed up coronavirus fight
22 April 2021 Syria receives batch of UN vaccines to speed up virus fight
22 April 2021 COVID-19: Syria gets 200,000 doses of AstraZeneca vaccine under COVAX scheme
22 April 2021 Syria gets 200,000 doses of AstraZeneca vaccine under COVAX scheme: U.N. officials
22 April 2021 Syria gets 200,000 doses of AstraZeneca vaccine under COVAX scheme - U.N. officials
22 April 2021 Syria Government Receives First COVAX Jabs
22 April Damascus receives first COVAX vaccines
22 April 2021 UK coronavirus variant cases soar in northeast Syria; vaccine yet to arrive
17 April 2021 Urgent call from Rojava authorities to prevent COVID-19 'catastrophe'
14 April 2021 Health committee: possible catastrophe amid no coronavirus vaccines
14 April 2021 Kurds impose 10-day curfew in NE Syria amid coronavirus wave
14 April 2021 Inside COVAX’s Mission to Save the World
13 April 2021 Keeping Syria polio-free requires sustained commitment
9 April 2021 The Falafel factor: Syria’s disappearing middle class and rising polarization
9 April 2021 World Health Day address by WHO Representative to Syria [EN/AR]
9 April 2021 Escape to death: Specter of suicide haunts Syrian people
7 April 2021 Syria: Schools and Universities to Be Shut Amid Rising Coronavirus Cases
6 April 2021 Covid overwhelms ICUs in Syrian capital
4 April 2021 Syria to Close Schools and Universities over Virus Surge
3 April 2021 Syria to close schools and universities over virus surge
3 April 2021 WHO defends planned COVID-19 vaccine dose allocation for Rojava
3 April 2021 Covid overwhelms ICUs in Syrian capital
3 April 2021 How COVID-19 has overwhelmed ICUs in Syrian capital Damascus
2 April 2021 COVID overwhelms ICUs in Syrian capital
2 April 2021 Coronavirus saturates intensive care units in Damascus
2 April 2021 Northwestern Syria gears up to start an anti-COVID vaccination campaign next May
2 April 2021 Damascus hospitals experience unprecedented pressure
27 Mar 2021 Over 50% Of Syria's Health Sector Destroyed, Needs Restoration - WHO Mission Head
24 Mar 2021 WHO To Oversee Vaccination Campaign In Syria; Here Is All That You Need To Know
23 Mar 2021 WHO will start COVID-19 vaccine applications in Idlib in April
23 Mar 2021 World Health Body Plans to Vaccinate 20% of Syrians in 2021
23 Mar 2021 World health body plans to vaccinate 20% of Syrians in 2021
23 Mar 2021 World health body plans to vaccinate 20% of Syrians in 2021
23 Mar 2021 World health body plans to vaccinate 20% of Syrians in 2021
23 Mar 2021 Taking Care of the Essentials | Translated Article from Russian
22 Mar 2021 COVID-19 Testing Capacity In Syria Still Low Leading To Concerns Over Disease Spread - MSF
20 Mar 2021 Rojava to receive 90,000 doses of coronavirus vaccine within weeks: WHO
18 Mar 2021 Syria to get first deliveries of COVAX vaccines within weeks
17 Mar 2021 Syria launches COVID vaccine drive as Israel questions swirl
17 Mar 2021 Syria to Get First Deliveries of COVAX Vaccines within Weeks
17 Mar 2021 Syria to get first deliveries of COVAX vaccines within weeks: WHO official
8 Mar 2021 I want to focus on HOPE | Translated Article from Russian
6 Mar 2021 Northern Syria awaits first doses while fighting COVID-19 vaccine rumors
28 Feb 2021 Syrian opposition gears up for coronavirus vaccine drive
25 Feb 2021 Syria govt says it has virus vaccine; doesn't say from where
23 Feb 2021 Syria approves Russia’s Sputnik Covid vaccine
23 Feb 2021 Syria's rebel-held northwest to get Covid vaccines by end March: WHO
23 Feb 2021 Vaccines to arrive in rebel-held areas of Syria next month: WHO
22 Feb 2021 Syria approves Russia's Sputnik Covid vaccine
22 Feb 2021 Idlib Health Directorate to start vaccinating people against Coronavirus next March
22 Feb 2021 Monopoly or competition between fuel companies in rebel-held Idlib?
17 Feb 2021 Only 20% of population to get COVID-19 vaccines—across three Syrian control territories
14 Feb 2021 Mystery surrounds the topic of providing COVID-19 vaccines | Al-Watan Article - English Translation
4 Feb 2021 WHO Deploying Teams Across Syria for COVID-19 Vaccination Program
4 Feb 2021 COVAX unveils plan for global distribution of 337M vaccines
3 Feb 2021 WHO: Vaccine In Syria May Only Cover 3% In 1st Phase
2 Feb 2021 Slow COVID-19 vaccine rollout expected in war- ravaged Syria (yahoo.com)
31 Jan 2021 WHO preparing to deliver vaccines across Syria from April despite conflict | Reuters
COVAX unveils plan for global- distribution of 337m vaccines
31 Jan 2021 https://www.diepresse.com/5930435/das-dilemma-der-hilfsorganisationen?from=rss
14 Jan 2021 Syria’s COVID-19 vaccine is expected by April, but fair access is not guaranteed - Syria Direct
31 Dec 2020 Idlib: COVID-19 and the oxygen bottleneck
17 Dec 2020 In syria, put humanitarian aid ahead of a political solution
9 Nov 2020 Coronavirus opens new door to corruption in Syria
28 Oct 2020 Syria is the deadliest place for aid workers, and there is little hope for change
27 Oct 2020 MOH FB page about UHC workshop
27 Oct 2020 Arnous, WHO discuss cooperation to confront Corona epidemic – Syrian Arab News Agency (sana.sy)
27 Oct 2020 Syria, WHO to enhance cooperation in medical domain – Syrian Arab News Agency (sana.sy)
27 Oct 2020 WHO delegation visits University Children Hospital in Damascus – Syrian Arab News Agency (sana.sy)
26 Oct 2020 MOH FB page on RD visit to Hama
25 Oct 2020 MOH FB page on RD and WR meeting with Minister of Health
25 Oct 2020 Mikdad: Unilateral coercive measures imposed on Syria contradict UN Charter – Syrian Arab News Agency
25 Oct 2020 Syria receives three ambulances and five mobile clinics offered by WHO – Syrian Arab News Agency (sana.sy)
MOI FB page on receiving ambulances and mobile clinics
24 Oct 2020 Shipment of medical assistance from World Health Organization to Syria – Syrian Arab News Agency (sana.sy)\
21 Oct 2020 Breast cancer patients have determination and patience amid limited medical services in rebel-held Idlib
13 Oct 2020 Coronavirus: Syria government 'prepares for virus second wave'
7 Oct 2020 Busy Damascus cemetery points to higher pandemic death toll in Syria
26 Sept 2020 Rising COVID-19 cases put additional pressure on medical facilities in northern Syria
22 Sept 2020 From the U.S. to Syria, a Doctor Smuggles Lifesaving Equipment
14 Sept 2020 Article Link
Wheat and Fuel Shortages Overwhelm Syria
7 Sept 2020 We Are All Scared, All The Time': Syrian Doctors Can't Talk About The Coronavirus
7 Sept 2020 Syrian Kurdish authorities to free 25,000 Syrians from camp for Islamic State supporters (telegraph.co.uk)
1 Sept 2020 The hospitals where Covid-19 sufferers wait for others to die before they can be put on a respirator
28 Aug 2020 Coronavirus in camps of northwestern Syria despite previous warnings… Who is to blame?
27 Aug 2020 UN official: COVID cases likely far higher than Syria says
29 Aug 2020 Syria's coronavirus crisis becoming clear in Damascus
31 July 2020 Syria Is Overwhelmed By Coronavirus As Govt Conceals Outbreak, Health Worker Says
7 July 2020 INTERVIEW Head of WHO office in Syria: "If people are left alone, give them peace, they are ready to create"
1 July 2020 Redefining global care: Duty, service and choices made in the time of COVID-19 (who.int)
29 June 2020 ‘God forbid’ COVID-19 reaches Syria’s camps, warns WHO medic
11 May 2020 Factories In Syria Ramp Up Production Of Hydroxychloroquine Amid Soaring Demand
28 April 2020 Syria: Aid Restrictions Hinder Covid-19 Response
9 April 2020 Syria’s shattered health service left exposed as coronavirus spreads
30 March 2020 Coronavirus: Syria reports first death amid warning of spread
Summary of key indicators reported through the 4Ws
2021
Summary of humanitarian response plan key indicators reported through the 4Ws, August 2021
Summary of humanitarian response plan key indicators reported through the 4Ws, May 2021
Summary of humanitarian response plan key indicators reported through the 4Ws, April 2021
Summary of humanitarian response plan key indicators reported through the 4Ws, March 2021
Summary of humanitarian response plan key indicators reported through the 4Ws, February 2021
Summary of humanitarian response plan key indicators reported through the 4Ws, January 2021
2020
Summary of humanitarian response plan key indicators reported through the 4Ws, annual 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, December 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, November 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, October 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, September 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, August 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, July 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, June 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, May 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, April 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, March 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, February 2020
Summary of humanitarian response plan key indicators reported through the 4Ws, January 2020

Summary of humanitarian response plan key indicators reported through the 4Ws, December 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, November 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, October 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, September 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, August 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, January–June 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, July 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, June 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, May 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, first quarter 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, April 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, March 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, February 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, January 2019
Summary of humanitarian response plan key indicators reported through the 4Ws, October 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, September 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, August 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, July 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, June 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, May 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, April 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, January-March 2018
Summary of humanitarian response plan key indicators reported through the 4Ws, January-December 2017
Summary of humanitarian response plan key indicators reported through the 4Ws, January-November 2017
Summary of humanitarian response plan key indicators reported through the 4Ws in October 2017
Summary of humanitarian response plan key indicators reported through the 4Ws in September 2017
Summary of key performance indicators for whole of Syria
2021
WHO summary of key indicators Whole of Syria, January to June 2021
WHO summary of key indicators Whole of Syria, June 2021
WHO summary of key indicators Whole of Syria, May 2021
WHO summary of key indicators Whole of, April 2021
WHO summary of key indicators Whole of Syria, March 2021
WHO summary of key indicators Whole of Syria, February 2021
WHO summary of key indicators Whole of Syria, January 2021
2020
WHO summary of key indicators Whole of Syria, Annual 2020
WHO summary of key indicators Whole of Syria, December 2020
WHO summary of key indicators Whole of Syria, November 2020
WHO summary of key indicators Whole of Syria, October 2020
WHO summary of key indicators Whole of Syria, September 2020
WHO summary of key indicators Whole of Syria, August 2020
WHO summary of key indicators Whole of Syria, July 2020
WHO summary of key indicators Whole of Syria, June 2020
WHO summary of key indicators Whole of Syria, May 2020
WHO summary of key indicators Whole of, April 2020
WHO summary of key indicators Whole of Syria, March 2020

WHO summary of key indicators Whole of Syria, September 2019
WHO summary of key indicators Whole of Syria, August 2019
WHO summary of key indicators Whole of Syria, first half 2019
WHO summary of key indicators Whole of Syria, July 2019
WHO summary of key indicators Whole of Syria, June 2019
WHO summary of key indicators Whole of Syria, May 2019
WHO summary of key indicators Whole of Syria, April 2019
WHO summary of key indicators Whole of Syria, First quarter 2019
WHO summary of key indicators Whole of Syria, March 2019
Update on COVID-19 vaccination in Syria, 17 March 2021

17 March 2021 - COVAX is the vaccines pillar of the ACT-Accelerator [1], an instrument jointly convened by the Coalition for Epidemic Preparedness and Innovations (CEPI), WHO and the Vaccine Alliance (GAVI) to speed up the search for an effective vaccine for all countries; support the building of manufacturing capabilities; and buy supply ahead of time so that 2 billion doses can be fairly distributed globally by the end of 2021.
Under the COVAX facility, Syria is one of the 92 countries eligible for advanced market distribution (AMD) of COVID-19 vaccines.
In coordination with GAVI, WHO and UNICEF are providing detailed technical assistance to the national health authority in Syria and established committees such as the high level National Coordination Committee (NCC), the National COVID-19 Technical Advisory Group (CTAG) and the Inter-Agency Coordination Committee (ICC).
Vaccine Request Form (VRF)
Part A of the COVAX COVID-19 vaccine application document was signed by the Syrian Minister of Health and sent to GAVI on 15 December 2020. On 27 January 2021, the Syrian Prime Minister declared the Syrian government’s approval of the COVAX vaccine initiative. Part B of the vaccine application was signed and sent to GAVI on 3 February.
On 3 February 2021 GAVI acknowledged and expressed the intent to provide initially 1,020,000 doses of Astra Zenica Serum Institute of India (AZ SII) vaccines, to cover the first 3% of the population (targeted high-risk groups), including the population in northeast Syria. Additional 336,000 doses were intended for northwest Syria.
On 15 February 2021 WHO granted Emergency Use Listing (EUL) for the AstraZeneca AZD1222 vaccine produced by the Serum Institute of India vaccine (SII-AZ).
Later, the allocation of the AZ SII vaccine was confirmed by GAVI through May 2021 as 912,000 doses for Phase 1 in addition to 224,000 doses of the same vaccine to the Phase 1 vaccine administration in the northwest. The month of June is not included in these allocations, which is the main reason that the quantities are less than the intended indicative allocation previously communicated.
This amount may only cover around 4% of the total population. Indicative distribution is based on current communication of estimated vaccine availability from manufacturers. It is likely that distribution will need to be adjusted in light of circumstances that are difficult to anticipate and variables that are constantly evolving.
The Indemnity and Liability agreement with the manufacturer was signed by the Ministry of Health and the corresponding manufacturer.
Regular daily meetings have been held since the beginning of 2021 by three vaccine-related committees (the NCC, the CTAG and the ICC), with WHO and UNICEF present at the ICC meetings. The WHO COVID-19 Vaccine Introduction Readiness Assessment Tool (VIRAT/VRAF 2.0) has been used to update national readiness status on a monthly basis, with the most recent update submitted on 23 February 2021.
Coordination framework
The NCC, the CTAG, the ICC and 10 technical sub-committees have been fully operational, with clear terms of reference, since the end of January 2021.
1. National readiness assessment
The updated VIRAT/VRAF 2.0 includes assessment of planning and coordination, budgeting, regulatory measures, prioritization, targeting and surveillance, service delivery, training and supervision, monitoring and evaluation, vaccine cold chain, logistics, safety surveillance, and demand generation and communication. It covers a set of 50 key operational activities. Syria has been using this tool according to the following timetable:
The first update was submitted at end November 2020;
The second update was submitted on 14 January 2021;
The third update was endorsed by the Ministry of Health on 20 January 2021;
The fourth and final update was submitted on 23 February 2021.
2. Establihsment of taskforces
To bridge gaps in capacity and planning and implementation, and to ensure preparedness for key areas of vaccine introduction, 10 sub-committees have been formed as the technical part of the CTAG committee. These sub-committees include WHO and UNICEF focal points, and meet regularly to update the VIRAT/VRAF 2.0 work and prepare necessary materials for the NDVP.
WHO and UNICEF are holding monthly coordination meetings, the first of which took place on 14 February 2021.
3. Population prioritization
The priority categories identified in Part A of the COVID-19 vaccine application document are based on the CTAG’s recommendations, the SAGE values framework and the COVAX facility fair allocation prioritization roadmap. For Syria, the following high-risk groups were agreed upon as targets under COVAX:
The health workforce (including frontline social workers and teachers): 3% of the population
Older adults (>55-years): approximately 13% of the population
People with chronic diseases: 5% of the population.
At present, national authorities collect and consolidate population data at national and governate levels (including from the Ministry of Planning, the Central Statistics Office and the Syndicate of Doctors and Health Workers). This data includes all 14 governorates of Syria, including northwest and northeast Syria. The Ministry of Health relies greatly on existing mechanisms and modalities related to previous experiences of successful routine immunization activities across these governorates (see section 7 for more on northwest Syria).
As decided in a meeting on 7 February 2021, the vaccination of the first 20% of the population will be carried out in three phases as outlined below, with doses adjusted according to quantities made available by COVAX and updates to population figures.
|
Phase |
Groups |
Estimated number of people vaccinated* to be adjusted as per available vaccine allocation |
|
Phase One |
All health workers |
190 000 |
|
Older group (55 years or more) |
485 450 |
|
|
Phase Two |
Rest of the older group |
1 540 900 |
|
Persons with comorbidities |
1 125 750 |
|
|
School teachers |
302 827 |
|
|
Other essential workers |
858 073 |
|
|
Phase Three |
|
To be determined |
4. Pre-registration mechanism
In collaboration with the committees, WHO is supporting the development and introduction of an automated pre-registration platform and reporting mechanism. Pre-registration will help identify target groups and aid vaccine distribution. This approach will not, however, be the only method for pre-registration, and exemptions are being factored in for some cases.
5. Service delivery mechanisms
Under current plans, 76 hospitals will be used as service delivery points to provide vaccinations, along with 101 primary health care facilities all over the country. Services will be provided by trained hospital teams and routine immunization personnel in mobile teams. This number of facilities and associated teams is preliminary and subject to change based on ongoing microplanning. Each hospital will have three or more teams assigned to microplanning for each phase of the campaign.
Implementation across northeast Syria will follow the current experiences of the Expanded Programme on Immunization (EPI) microplanning through 17 fixed facilities (hospitals and PHC centres) and 105 mobile teams. Formal and informal settlements will be targeted in the same way. Microplanning will also cover the populations of camps across northeast Syria. The first batch of vaccines will target eligible high-risk members of the health workforce and frontline humanitarian workers regardless of location. WHO will support transport of the vaccine inside Syria, including to northeast Syria, and coordinate mobile activities with different stakeholders based on existing operations.
6. Monitoring and evaluation
Currently, for the national immunization programme, the Ministry of Health is using aggregate reporting system where administered doses are recorded by age and gender, tallied along key dimensions, and reported up the health system, often using a mix of digital and paper tools. A similar approach is being used also by the Syria Immunization Group in northwest Syria.
After the immunization campaign concludes, independent monitors from universities, health colleagues and national NGO partners will be deployed to ensure the vaccination campaign coverage. This approach will be used for the COVID-19 vaccination. Furthermore, a more active form of monitoring and evaluation that covers the pre-, intra and post- implementation of the vaccination activity at the field level, including assigning a third party for independent M&E is planned by WHO, UNICEF and MOH.
Paper-based records will be updated to reflect COVID-19 vaccination status to:
provide proof of vaccination for individual’s travel, educational or occupational purposes;
establish vaccination status in coverage surveys;
provide vaccination information in case of an AEFI or in case of a positive COVID-19 test; and
provide a useful vaccination card for adults and older adults to which COVID-19 vaccines and other recommended vaccines can be added and guidance on any doses required to complete vaccination course can be found.
During the vaccination campaigns, monitoring activities are conducted through different strata of supervision from the central, governorate, district team supervisors.
For the COVID-19 vaccination, a team consisting of representatives from MOH, WHO and UNICEF is formed and working on a monitoring and evaluation plan for government-controlled areas and northeast Syria. The WHO monitoring guide for COVID-19 vaccination has highlighted the potential sources for COVID-19 vaccination data through Health Information System, facility reports, electronic immunization registers and surveillance data for AEFI/AESI.
In nortwest Syria, WHO in partnership with UNICEF and COVID-19 taskforce is updating Monitoring and Evaluation tools and strategies for the COVID-19 vaccination campaigns. In northwest Syria the evaluation process will be implemented through third party independent monitors who will be deployed to ensure the vaccination campaign process in 3 phases - pre, intra and post campaign monitoring.
7. Risk communication and demand generation
WHO and UNICEF are working in close cooperation with the Ministry of Health to develop the COVID-19 vaccination media campaign, which includes capacity building workshops for journalists, health educators and community influencers. It also entails the development of a full media package (TV and radio spots, social media messages, billboards, posters, flyers, etc.) to be implemented nationally.
Based on learnings from previous COVID-19 prevention and response interventions, five strategies will guide the introduction of COVID-19 vaccines at national and state level. These are as follows:
advocacy to gain commitment and garner support for rollout the new COVID-19 vaccine;
capacity building to enhance communication and community mobilization skills of target workers (including heath care providers, health education officers, NGOs, etc.);
media engagement and social media campaigns to promote balanced, evidence-based discourse on COVID-19 vaccines and the vaccination process (these campaigns will set out to manage demand and vaccine hesitancy, build trust and manage misinformation and rumours);
community engagement; providing prompt, simple, focused communication to communities in order to manage expectations and hesitancy concerns; and
crisis communication, including around adverse events following immunization (AEFI). Rapid responses will be prepared to manage crisis situations arising from demand and vaccine hesitancy.
8. Northwest Syria
WHO Syria maintains a direct day-to-day dialogue with the WHO hub in Gaziantep, Turkey. Together with UNICEF, the hub has submitted a COVAX application for implementation of COVID-19 vaccinations based on the existing immunization programme in northwest Syria.
As previously mentioned, northwest Syria has been allocated 224 000 doses of the AstraZeneca AZD1222 vaccine through May 2021.
Target groups were prioritized based on a series of discussions between involved parties, and include health care workers (3%); elderly people aged 60 and above (7.5%); and people aged 20-59 with special conditions, such as immune-compromised people and those with chronic illnesses (9.5%). The GAVI letter received on 3 February 2021 expresses the intent to allocate sufficient vaccines to cover an initial 3% of the population with AZ SII vaccines (an indicative amount of 336 000 doses).
The following activities have been undertaken in northwest Syria:
WHO and partners have finalized the first draft of an estimated budget for the COVAX vaccination campaign that covers different possible scenarios.
WHO and partners have finalized the development of the National Deployment and Vaccination Plan for northwest Syria. This was submitted to the WHO Regional Office for the Eastern Mediterranean and presented to, and approved by, the Regional Review Committee (RRC) on 16 February 2021.
Partners are developing standard operating procedures (SOPs), formats and channels for the vaccination campaign and reviewing training materials for the context of northwest Syria.
The Health Cluster and partners are supporting estimations of the number of priority health workers in the field, with the aim of improving the accuracy of estimated numbers.
9. Development of the National Deployment and Vaccination Plan (NDVP)
The NDVP was submitted on 9 February, resubmitted after comments on 19 February, and approved on 22 February. Two trained WHO consultants (international, national) are currently supporting sub-committees at the Ministry of Health that are working on microplanning.
10. Guidelines, forms, reporting materials
Work is ongoing to develop the following resources:
vaccination cards, vaccination registers and reporting forms;
a monitoring and supervision checklist;
guidelines, checklists and reporting forms for AEFI;
updated COVID-19 reporting forms that include vaccination;
infection prevention and control (IPC) and waste management protocols; and
communication materials.
11. Cold chain
A nationwide cold chain inventory has been finalized and gaps for different scenarios have been identified. Training-of-trainers for cold chain and logistics officers has been conducted at central level. UNICEF has contracted two consultants to review and enhance this component, and the cold chain application was submitted on 21 February 2021. WHO’s Gaziantep hub and partners have developed the cold chain equipment (CCE) application for northwest Syria, which was submitted on 15 February 2021.
12. Vaccination in high-risk areas
The Ministry of Health has decided to use a combination of fixed facilities and mobile teams to vaccinate health workers in hard-to-reach areas. Microplanning will include high-risk groups and high-risk areas and possible mechanisms through which to reach them, based on experience and learning from the EPI. Population figures for camps and settlements are being collected for review and the necessary endorsement regardless of the areas of control (including in northeast Syria).
Next steps and key areas
CTAG meetings will be held to approve the decisions of the technical sub-committees and finalize microplanning. This will include identifying the targeted populations and which vaccination point will cover them; identifying high-risk groups and ways and mechanisms to reach them; and agreeing the number of vaccination days for each team and the number of team members and staff included at each level.
The development of guidelines, protocols, checklists, and reporting forms will then be finalized, and planning will be done for an electronic reporting system to report vaccinations and AEFI cases (discussions on streamlining support for this system are ongoing between the Ministry of Health and WHO. A timeline for all planned activities will be set and ongoing high-level coordination will begin, with the goal of vaccine rollout using a whole-of-Syria approach.
Training-of trainers, cascaded trainings and orientation meetings have started on 17 March 2021 and will continue at a provincial level.
Throughout this process WHO and UNICEF will continue to work closely with the Ministry of Health in Syria.
13. Challenges
WHO is committed to making every effort to combat COVID-19 in Syria and make vaccines available to the Syrian people.
There are, and will be, many “unknowns” as we move forward. It is important to know that while at present COVAX allocation is the best means of securing vaccines across Syria, there are also discussions at global level to avail a “humanitarian buffer” of vaccines, which can remain contingent once made available.
Among the many unknowns that could influence vaccine deployment are the following issues:
unpredictable manufacturing and global vaccine availability: the exact arrival date of the first batch of vaccine allocated to Syria is still not defined;
the instability of the security situation on the ground;
the fact that COVAX commitment is not currently ensured beyond the initial 3%;
the fact that options to secure vaccines may be limited in the long run, resulting in increased humanitarian needs;
the fact that current mutations and variants of the COVID-19 virus circulating in Syria are not known, making it difficult to predict or prove the efficacy of the introduced vaccines (WHO has sent samples for sequencing at the WHO Regional Reference Labs, so this may improve);
uncertain and unpredictable availability of funding to support rollout of COVID-19 vaccination;
the fact that continuity of cross-border operations in northwest Syria depends heavily on a UN Security Council Resolution that currently only lasts until July 2021; and
the need for contingency planning to ensure continuity of care for Q3 and Q4 of 2021 with COVAX vaccination.
14. Vaccine introduction costs
The estimated operational cost of the first phase of vaccine rollout under COVAX, targeting 3% of the population (front-line health workers and social workers) during the first and second quarter of 2021, is US$7 million. This includes US$4.5 million for areas under the control of the Government of Syria and northeast Syria, and US$2.5 million for northwest Syria.
The second phase of vaccine rollout will target the next 17% of the population and will include the elderly and those with chronic diseases. This will take place in the third and fourth quarter of 2021. The estimated gap in operational costs is US$32 million, including US$24.3 million for areas under the control of the Government of Syria and northeast Syria, and US$7.5 million for northwest Syria.
The table below outlines the operational cost of vaccinating 20% of the population in government controlled areas and northeast Syria, and the agreed cost sharing between WHO and UNICEF.
Estimated Budget Breakdown for Vaccine Introduction Costs to Cover 20% of the Population by end of December 2021
|
Budget summary for 2 Rounds |
Damascus |
Gaziantep (cross border) |
Total |
||
|
Cost be covered by WHO CO |
Cost to be covered by UNICEF |
Cost to be covered by WHO |
Cost to be covered by UNICEF |
||
|
Human resources and incentives |
$8,773,424.00 |
$1,066,317.00 |
$5,298,979.20 |
$0.00 |
$15,138,720.20 |
|
Training |
$707,323.00 |
$99,523.00 |
$358,137.60 |
$0.00 |
$1,164,983.60 |
|
Meetings |
$444,299.00 |
$0.00 |
$528,379.92 |
$0.00 |
$972,678.92 |
|
Cold chain, supplies and Logistic |
$2,677,852.00 |
$2,903,453.00 |
$752,077.92 |
$0.00 |
$6,333,382.92 |
|
Transportation |
$4,023,314.00 |
|
$1,526,804.40 |
$0.00 |
$5,550,118.40 |
|
Evaluation & Monitoring |
$1,878,748.00 |
$0.00 |
$662,833.00 |
$0.00 |
$2,541,581.00 |
|
Social mobilization |
$952,068.00 |
$5,317,619.00 |
|
$500,000.00 |
$6,769,687.00 |
|
Supporting management cost for contracted NGOs |
$0.00 |
$0.00 |
$372,787.68 |
$0.00 |
$0.00 |
|
Grand Total |
$19,457,028 |
$9,386,912 |
$9,499,999.72 |
$500,000 |
$38,843,941 |
Previous updates
Update on COVID-19 vccination in Syria, 1 March 2021
Update on COVID-19 vccination in Syria, 16 February 2021
Update on COVID-19 vaccination in Syria, 1 March 2021
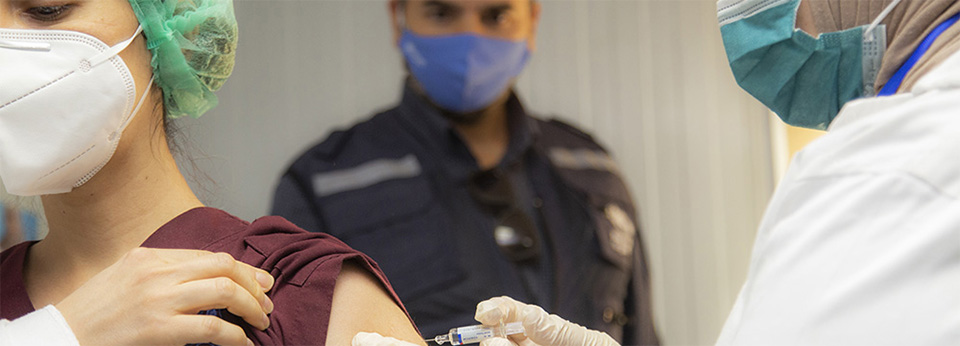 Photo credit: WHO/Syria
Photo credit: WHO/Syria
1 March 2021 - COVAX is the vaccines pillar of the ACT-Accelerator [1], convened by the Coalition for Epidemic Preparedness and Innovations (CEPI), GAVI - the Vaccine Alliance (GAVI) and WHO. Syria is one of the 92 countries eligible for Advanced Market Distribution (AMD) of COVID-19 vaccines under the COVAX facility, a partnership between the World Health Organization (WHO), the Coalition for Epidemic Preparedness Innovations (CEPI), and the Global Alliance for Vaccines and Immunization (GAVI).
In coordination with GAVI, WHO and UNICEF continue providing detailed technical assistance to and sharing guidelines with the national health authority and established committees, such as the high level National Coordination Committee, CTAG-national COVID-19 Technical Advisory Group and Inter-agency Coordination Committee.
Vaccine Request Form (VRF) - Part A of the COVID-19 Vaccine Application document was signed by the Minister of Health and sent to GAVI on 15 December 2020. On 27 January 2021, the Syrian Prime Minister declared the Government’s approval of the vaccine initiative through the COVAX facility. Part B was signed on 3 February and sent to GAVI.
GAVI, in return, on 3 February 2021 has acknowledged and expressed the intent to provide initially one million twenty thousand doses of Astra Zenica Serum Institute of India (AZ SII) vaccines, to cover the first 3% of the population (targeted high-risk groups), including the population in the north-east.
The National Deployment and Vaccination Plan (NDVP) was submitted on 9 February 2021, and re-submitted, after review, on 16 February, and approved on 19 February 2021. Cold chain application is submitted on 21 February 2021.
As per GAVI, the first allocation of vaccines is anticipated by the end of the first quarter or within the second quarter 2021 at the latest, after signing of the indemnity document with the manufacturer and confirming operational readiness.
Regular daily meetings are held in the past weeks since the beginning of 2021 by the 3 vaccine-related committees NCC, CTAG and ICC, the meetings of the latter being attended by WHO and UNICEF.
The Vaccine Introduction Readiness Assessment Tool (VIRAT tool) has been used to update the readiness status on a monthly basis, the final update submitted on 23 February 2021.
1. Coordination framework established
The following coordination committees are in existence, with clear TORs (terms of reference) and fully operational since end of January 2021, along with 10 technical sub-committees:
- NCC - National Coordination Committee,
- CTAG - COVID-19 Technical Advisory Group and
- ICC - Inter-Agency Coordination Committee
2. National readiness assessment
VIRAT/VRAF version updated tool includes planning and coordination, budgeting, regulatory, prioritization, targeting and surveillance, service delivery, training and supervision, monitoring and evaluation, vaccine cold-chain, logistics, safety surveillance, and demand generation and communication – a set of 50 key operational activities:
- First update was submitted at the end of November 2020.
- Second update was submitted on 14 January 2021.
- Third update was endorsed by the Ministry of Health on 20 January 2021.
- Fourth and final update was submitted on 23 February 2021.
3. Establishment of taskforces
To bridge capacity and planning and implementation gaps and to ensure preparedness regarding key areas of vaccine introduction, 10 sub-committees were formed as the technical part of the cTAG committee (WHO and UNICEF as focal points are included). Meetings take place regularly to update the VIRAT and to prepare the needed materials for the national vaccine deployment plan.
WHO and UNICEF also instituted monthly coordination meetings with the first meeting held on 14 February 2021.
4. Population prioritization
The priority categories identified in Part A are based on the National Technical Advisory Group recommendation, SAGE values framework and the COVAX facility fair allocation (prioritization roadmap). The following high-risk groups were agreed upon as a target under COVAX:
- Health workforce (including front line social workers and teachers) - 3% of population;
- Older adults >55-year population - about 13% of population;
- People with chronic diseases - 5% of population.
At present, the national authorities collect and consolidate population data (including from the Ministry of Planning, Central Statistics Office, Syndicate of Doctors and Health Workers at national and governate levels). The population data includes all 14 governorates of Syria, the north-west and north-east Syria. The Ministry of Health highly relies on the existing mechanisms and modalities related to the previous experience of the successful routine immunization activities across these governorates (see section 7 for more on north-west Syria).
As per the meeting conducted on 7 February 2021, the vaccination of planned 20% of the population will be carried out in 3 phases as follows, with the doses adjusted as per quantities made available by COVAX and adjusted population figures:
|
Phase |
Groups |
Estimated number of people vaccinated* to be adjusted as per available vaccine allocation |
|
Phase One |
All health workers |
190 000 |
|
Older group (55 years or more) |
485 450 |
|
|
Phase Two |
Rest of the older group |
1 540 900 |
|
Persons with comorbidities |
1 125 750 |
|
|
School teachers |
302 827 |
|
|
Other essential workers |
858 073 |
|
|
Phase Three |
|
To be determined |
5. Pre-registration mechanism
WHO is supporting the development and introduction of the pre-registration automated platform and reporting mechanism working together with the existing committees. The pre-registration will support in identifying target groups and vaccine distribution. However, this modality will not be the only way for the pre-registration and the exemptions are being factored in for some cases.
6. Service delivery mechanisms
At this stage, 76 hospitals used as service delivery points are planned to provide vaccination, on top of 101 primary health care facilities all over the country. This number of facilities and teams is preliminary and is subject to change based on the ongoing microplanning. Each hospital will have 3 or more teams assigned to the microplanning for each phase of the campaign. Services will be provided by the trained hospital teams and by routine immunization personnel as part of the mobile teams.
The implementation across the north-east will follow the current experience of the Expanded Programme on Immunization (EPI) microplanning through the 17 fixed facilities (hospitals and PHC centres) and 105 mobile teams. Formal and informal settlements will be targeted as well through the same modalities. The microplans will equally cover the population of camps across north-east Syria. The first batch of vaccines will target eligible high-risk health workforce and frontline humanitarian workers, regardless of the location. WHO will support the transport of the vaccine inside Syria including to the north-east Syria and coordinate the mobile activities on the ground with different stakeholders, based on the existing operations.
7. Northwest Syria
WHO Syria maintains a direct day-to-day dialogue with WHO Turkey. WHO Gaziantep office, together with UNICEF, has submitted COVAX application relying on the implementation based on the currently existing immunization programme modality in northwest Syria (NWS).
The target groups were prioritized based on series of discussions among the parties involved and include health care workers (3%), the elderly aged 60 and above (7.5%) and people in the age group 20-59 with special conditions such as immune-compromised persons and persons with chronic illnesses (9.5%). The GAVI letter received on 3 February 2021 expresses the intent to allocate vaccines to cover the initial 3% of the population with AZ SII vaccines (indicative, 336 000 doses).
The following activities were undertaken:
- The Technical assistance plan for COVAX was developed and submitted on 28 February 2021.
- The Vaccine Request Form (Plan A) was developed, submitted in 7 December 2020.
- WHO and partners have finalized the first draft of the COVAX vaccination campaign plan and budget.
- WHO and partners finalized the development of the National Deployment and Vaccination Plan (NDVP) for north-west Syria; the plan was submitted in time and approved on 17 Febrary 2021.
- WHO and partners developed a cold chain equipment (CCE) application package submitted on 15 February 2021.
- Partners are developing SOPs, formats and channels of the vaccination campaign, reviewing training materials for the context of north-west Syria.
- Health cluster and partners support estimation of the priority health workers working in the field aiming to get better estimated numbers.
8. Development of the national deployment and vaccination plan
- NDVP (submitted on 9 February and re-submitted, after comments, on 19 February) was approved on 22 February.
- 2 trained WHO consultants (international, national) are currently supporting the work of the sub-committees at the Ministry of Health on microplanning.
9. Guidelines, forms, reporting materials
Work is ongoing to develop the following:
- Vaccination cards, vaccination registers, reporting forms;
- Monitoring and supervision checklist;
- AEFIs guidelines, checklist and reporting forms;
- Updating COVID-19 reporting forms to include vaccination;
- IPC and waste management protocols;
- Communication materials.
10. Cold chain
- Nationwide cold chain inventory was finalized and gaps for different scenarios were identified.
- UNICEF contracted two consultants to review and enhance this component. The cold chain application is submitted on 21 February 2021.
- WHO Gaziantep and partners developed the cold chain equipment (CCE) application for north-west Syria, which was submitted on 15 February 2021.
11. Vaccination in high-risk areas
- The Ministy of Health decided to use a combination of fixed facilities and mobile teams to vaccinate health workers in hard-to-reach areas.
- The micro plans will include the high-risk groups and high-risk areas and the possible mechanism to reach them (as per EPI experience).
- Population figures of camps and settlements are collected regardless of the areas of control (including in north-east Syria) for the review and the necessary endorsement.
Next steps and key areas
- cTAG meetings to approve what was agreed by sub-committees.
- Finalize microplanning which includes:
identifying the targeted population and by which vaccination point they will be covered.
identifying high-risk groups, ways and the mechanisms of reaching them.
agreeing on the number of vaccination days by each team.
agreeing on the number of team members and staff included at each level.
- Finalize the developments of guidelines, protocols, checklists, reporting forms for printing.
- Plan for an electronic reporting system to report on vaccinations and AEFI cases (ongoing discussion between MOH and WHO to streamline support).
- Develop the needed operational cost, cost of vaccine supplies and the possible source of funds using costing tool.
- Set a timeline for all the planned activities before the vaccine introduction.
- Coordinate at the high-level coordination and have a dialogue to continue planning and vaccine roll out using Whole of Syria approach.
- Get the Indemnity and Liability agreementsigned between the government and the manufacturer.
WHO and UNICEF continue to work closely with the Ministry of Health in Syria.
12. Challenges
While we are committed and put our efforts to combat COVID-19 and make vaccines available to the Syrian people, there are many ‘unknowns’ as we move forward. It is important to know that at present COVAX allocation is our best enabler to secure vaccines across Syria. There are discussions at the global level to avail ‘humanitarian buffer’ of vaccines, which can remain contingent once made available.
The “unknowns” that may influence vaccine deployment include:
- Manufacturing and global vaccine availability – the arrival of the first batch of vaccine allocated to Syria is still not defined;
- The security situation on the ground;
- COVAX commitment beyond the initial 3% is not ensured;
- Options to secure the vaccines, in the long run, may be limited resulting in increased humanitarian needs;
- The current mutations and variants of the COVID-19 virus circulating in Syria are not known, making it difficult to prove the efficacy of the introduced vaccines (*however, WHO has sent samples for sequencing at the WHO Regional Reference Labs);
- Availability of funding for COVAX Vaccine to support the roll out of COVID-19 vaccination;
- Continuity of the cross-border operations in north-west Syria heavily depends on the UN Security Council Resolution (scheduled July 2021) and contingency planning to ensure continuity of care for Q3 and Q4 of 2021 with COVAX vaccination.
13. Vaccine introduction costs
Estimates for the operational costs for the first phase of the vaccine roll-out under COVAX, targeting 3% of the population (front-line health workers and social workers), during the first and second quarter: US$ 7 000 000 (US$ 4.5 million for the areas under the control of Government of Syria and the north-east Syria, and US$ 2.5 million for northwest Syria areas).
The second phase of the vaccine roll-out will target the next 17% of the population and include the elderly and those with chronic diseases; it will take place in the third and fourth quarter. Estimated gaps in operational costs: US$ 32 000 000 (US$ 24.3 million for areas under the control of Government of Syria and northeast Syria, and US$ 7.5 million for northwest Syria.
Previous updates
Update on COVID-19 vccination in Syria, 16 February 2021
Update on COVID-19 vaccination in Syria
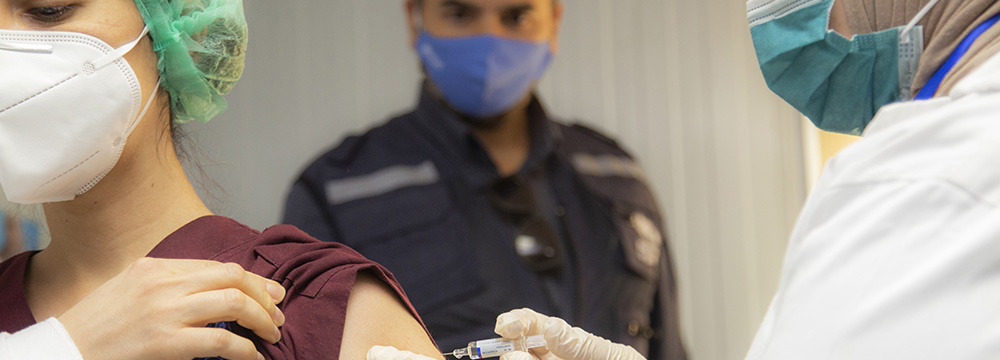
17 February 2021 – COVAX is the vaccines pillar of the ACT-Accelerator [1], convened by the Coalition for Epidemic Preparedness and Innovations (CEPI), GAVI - the Vaccine Alliance (GAVI) and WHO.
Syria is one of the 92 countries eligible for Advanced Market Distribution (AMD) of COVID-19 vaccines under the COVAX facility, a partnership between the World Health Organization (WHO), the Coalition for Epidemic Preparedness Innovations (CEPI), and the Global Alliance for Vaccines and Immunization (GAVI).
In coordination with GAVI, WHO and UNICEF continue providing detailed technical assistance to and sharing guidelines with the national health authority and established committees, such as the high level National Coordination Committee, CTAG-national COVID-19 Technical Advisory Group and Inter-agency Coordination Committee.
Vaccine Request Form (VRF) - Part A of the COVID-19 Vaccine Application document was signed by the Minister of Health and sent to GAVI on 15 December 2020. On 27 January 2021, the Syrian Prime Minister declared the Government’s approval of the vaccine initiative through the COVAX facility. Part B was signed on 3 February and sent to GAVI.
GAVI, in return, on 3 February 2021 has acknowledged and expressed the intent to provide initially one million twenty thousand doses of Astra Zenica SII (AZ SII) vaccines [2], to cover the first 3% of the population (targeted high-risk groups), including the population in the north-east.
The National Vaccine Deployment Plan (NDVP) was submitted on 9 February 2021, to which comments were provided on 16 February for further review and re-submission. As per GAVI, the first allocation of vaccines is anticipated by the end of the first quarter or within the second quarter 2021 at the latest, once the NDVP is endorsed after its technical review and signing of the indemnity document with the manufacturer.
Regular daily meetings are held in the past weeks since the beginning of 2021 by the 3 vaccine-related committees NCC, CTAG and ICC, the meetings of the latter being attended by WHO and UNICEF.
The Vaccine Introduction Readiness Assessment Tool (VIRAT tool) has been used to update the readiness status on a monthly basis, the next update is due on 21 February 2021.
1. Coordination framework established
The following coordination committees are in existence, with clear TORs and fully operational since end of January 2021:
- NCC - National Coordination Committee,
- CTAG - COVID-19 Technical Advisory Group and
- ICC - Inter-Agency Coordination Committee)
2. National readiness assessment
VIRAT/VRAF version updated tool includes planning and coordination, budgeting, regulatory, prioritization, targeting and surveillance, service delivery, training and supervision, monitoring and evaluation, vaccine cold-chain, logistics, safety surveillance, and demand generation and communication – a set of 50 key operational activities:
- First update was submitted at the end of November 2020.
- Second update was submitted on 14 January 2021.
- Third update was endorsed by the Ministry of Health on 20 January 2021.
- Fourth update is due on 21 February 2021.
3. Establishment of taskforces
To bridge capacity and planning and implementation gaps and to ensure preparedness regarding key areas of vaccine introduction, 10 sub-committees were formed as the technical part of the cTAG committee (WHO and UNICEF as focal points are included). Meetings take place regularly to update the VIRAT and to prepare the needed materials for the national vaccine deployment plan.
WHO and UNICEF also instituted monthly coordination meetings with the first held on 14 February 2021.
4. Population prioritization
The priority categories identified in Part A are based on the National Technical Advisory Group recommendation, SAGE values framework and the COVAX facility fair allocation (prioritization roadmap) and the following high-risk groups were agreed upon as a target under COVAX:
- Health workforce (including front line social workers and teachers), 3% of population;
- Older adults >55-year population, about 13% of population;
- People with chronic diseases, 5% of population.
At present, the national authorities collect and consolidate population data (including from the Ministry of Planning, Central Statistics Office, Syndicate of Doctors and Health Workers at national and governate levels). The population data includes all 14 governorates of Syria, the north-west and north-east Syria. The Ministry of Health highly relies on the existing mechanisms and modalities related to the previous experience of the successful routine immunization activities across these governorates (see section 7 for more on north-west Syria).
As per the meeting conducted on 7 February 2021, the vaccination of planned 20% will be carried out in 3 phases as follows, with the doses adjusted as per quantities made available by COVAX and adjusted population figures:
|
Phase |
Groups |
Estimated number of people vaccinated* to be adjusted as per available vaccine allocation |
|
Phase One |
All health workers |
190 000 |
|
older group (55 years or more) |
485 450 |
|
|
Phase Two |
Rest of the older group |
1 540 900 |
|
Person with comorbidities |
1 125 750 |
|
|
School teachers |
302 827 |
|
|
Other essential workers |
858 073 |
|
|
Phase Three |
|
To be determined |
5. Pre-registration mechanism
WHO is supporting the development and introduction of the pre-registration automated platform and reporting mechanism working together with the existing committees. The pre-registration will support in identifying target groups and vaccine distribution. However, this modality will not be the only way for the pre-registration and the exemptions are being factored in for some cases.
6. Service delivery mechanisms
At this stage, 76 hospitals used as service delivery points are planned to provide vaccination, on top of 101 primary health care facilities all over the country. This number of facilities and teams is preliminary and is subject to change based on the ongoing microplanning. Each hospital will have 3 or more teams assigned to the microplanning for each phase of the campaign. Services will be provided by the trained hospital teams and by routine immunization personnel as part of the mobile teams.
The implementation across the north-east will follow the current experience of the Expanded Programme on Immunization (EPI) microplanning through the 22 fixed facilities (hospitals and PHC centres) and 108 mobile teams. Formal and informal settlements will be targeted as well through the same modalities. The microplans will equally cover the population of camps across north-east Syria. The first batch of vaccines will target eligible high-risk health workforce and frontline humanitarian workers, regardless of the location. WHO will support the transport of the vaccine inside Syria including to the north-east Syria and coordinate the mobile activities on the ground with different stakeholders, based on the existing operations.
7. North-west Syria
WHO Syria maintains a direct day-to-day dialogue with WHO Turkey. WHO Gaziantep office, together with UNICEF, has submitted COVAX applications relying, for the implementation, on the currently existing immunization programme modalities in north-west Syria. The Vaccine Request Form (Part A) envisaged to cover up to 20% of the Syrian population residing in north-west Syria. The target groups were prioritized based on series of discussions among the parties involved and include health care workers (3%), the elderly aged 60 and above (7.5%) and people in the age group 20-59 with special conditions such as immune-compromised persons and persons with chronic illnesses (9.5%). The GAVI letter received on 3 February 2021 expresses the intent to allocate vaccines to cover the initial 3% of the population with AZ SII vaccines. More information will be provided at a later stage.
8. Development of the national deployment vaccination plan
- Trained WHO and national staff and consultants on the tools and methodology.
- Deployed 2 trained WHO consultants (international, national), who are finalizing the NDVP together with ICC and the sub-committees.
- The final plan is to be submitted on 9 February and to be endorsed by end of February 2021.
Meanwhile the team is engaged in refining the costs for NDVP and resource mobilization to meet the needs for vaccine implementation to cover initial 3% of the population with 2 doses of AZ SII. As per preliminary estimates the cost per person may exceed US$ 7-10, which may vary.
8. Guidelines, forms, reporting materials
Work is ongoing to develop the following:
- Vaccination cards, vaccination registers, reporting forms;
- Monitoring and supervision checklist;
- Adverse events following immunization guideline, checklist and reporting forms;
- Updating COVID-19 reporting forms to include vaccination;
- Infection prevention and control and waste management protocols;
- Communication materials.
9. Cold chain
- A nationwide cold chain inventory is being carried out. UNICEF contracted 2 international consultants to review and enhance this component.
- A desk review was conducted, the report is pending.
- A specific tool is being used to identify gaps and needs.
10. Vaccination in high-risk areas
- The national health authority took a decison to use a combimnation of fixed and mobile teams in the first phase to vaccinate all health workers, including those in hard-to-reach areas, private health facilities, humanitarian and UN frontline workforce.
- The micro plans will include the high-risk groups and high-risk areas and the possible mechanism to reach them (as per the national EPI programme experience).
- Population figures of camps and settlements are collected regardless of the areas of control (including north-east Syria) for the review and the necessary planning to include all high risk groups.
- All the above population figures and risk groups are being identified and quantified.
11. Next steps and key issues
- CTAG to review and approve recommendations from the sub-committees.
- Finalize microplanning for the vaccine introduction, which includes:
Identifying the targeted population groups and by which vaccination point they will be covered.
Identify high-risk groups, ways and the mechanisms of reaching them.
The number of vaccination days by each team.
The number of team members and staff included at each level.
- Finalize the developments of guidelines, protocols, checklists, reporting forms for printing.
- Plan roll out of training and distribution of forms, materials.
- Report and follow-up recommendations of the Quick Analysis of Cold Chain Inventory with the aim to capacitate beyond routine immunization program to meet the COVID-19 vaccine roll out requirements.
- Finalizing pre-registration automated platform and reporting mechanism to report on vaccinations and AEFI cases.
- Firm up the needed operation cost, cost of vaccine supplies and the sources of funds.
- Set a timeline for all the planned activities for vaccine introduction.
- High-level coordination to continue for proper planning and response across northwest Syria and northeast Syria, including WHO Syria and WHO Turkey.
12. Challenges
The vaccine introduction efforts have to deal with many ‘unknowns’ and it is important to know that at present COVAX allocation is our best enabler to secure vaccines across Syria. There are discussions at the global level to avail humanitarian buffer which can remain contingency once made available.
The “unknowns” that may influence vaccine deployment include:
- manufacturing and global vaccine availability – the arrival of the first batch of vaccine allocated to Syria is still not definite;
- security situation on the ground;
- COVAX commitment beyond initial 3% is not ensured;
- options to secure the vaccines in the long run may be limited resulting in increased humanitarian needs;
- the current mutations and variants of the COVID-19 virus circulating in Syria not known, making it difficult to efficacy of the introduced vaccines (*however WHO is working to send samples for sequencing at the regional reference laboratories).
13. Vaccine introduction costs
Estimates for the operational costs for the first phase of the vaccine roll-out under COVAX, targeting 3% of the population (front-line health workers and social workers), during the first and second quarter: US$7 000 000.
The second phase of the vaccine roll-out will target the next 17% of the population and include the elderly and those with chronic diseases; it will take place in the third and fourth quarter. Estimated gaps in operational costs: US$ 32 000 000.
Previous updates
Update on COVID-19 vaccination in Syria, 9 February 2021
Update on COVID-19 vaccination in Syria, 26 January 2021
[1] ACT-Accelerator or The Access to COVID-19 Tools (ACT) Accelerator, is a groundbreaking global collaboration to accelerate development, production, and equitable access to COVID-19 tests, treatments, and vaccines, more at https://www.who.int/initiatives/act-accelerator/
[2] AZ SII was approved by WHO and included in the Emergency Use Listing on 15 February 2021.
Update on COVID-19 vaccination in Syria, 9 February 2021
 Photo credit: WHO/Syria
Photo credit: WHO/Syria
9 February 2021 – COVAX is the vaccines pillar of the ACT-Accelerator[1], convened by the Coalition for Epidemic Preparedness Innovations (CEPI), the Global Alliance for Vaccines and Immunization (GAVI) and WHO.
Syria is one of the 92 countries eligible for Advanced Market Distribution (AMD) of COVID-19 vaccines under the COVAX facility, a partnership between the World Health Organization (WHO), the Coalition for Epidemic Preparedness Innovations (CEPI), and the Global Alliance for Vaccines and Immunization (GAVI).
In coordination with GAVI, WHO and UNICEF continue providing detailed technical assistance to and sharing guidelines with the national health authority and established committees, such as NCC- high level National Coordination Committee, CTAG-national COVID-19 Technical Advisory Group and ICC- Inter-agency Coordination Committee.










