M.T. Madkour,1 M.S. El Bokhary,2 H.I.Awad Allah,2A.A.Awad3 and H.F. Mahmoud4
التعرُّض البيئي للأسبستوس والعلاقة بين التعرُّض والاستجابة وبين ورم المتوسطة
مختار طه مدكور، محمود سري البخاري، هاله إبراهيم عوض الله، عبد الحميد عبد الحميد عوض، حسين فتحي محمود
الخلاصـة: أجرى الباحثون دراسة وبائية وبيئية في منطقة شبرا الخيمة بمدينة القاهرة الكبرى، حول العلاقة بين التعرُّض والاستجابة للأسبستوس (الأميانت) وبين ورم المتوسطة الـجَنْبَوي الخبيث. وقد أجرى الباحثون تنظيراً شعاعياً لـ 487 شخصاً معرَّضين مهنياً للأسبستوس ، و2913 شخصاً معرَّضين بيئياً له، ومجموعة شاهدة مكوَّنة من 979 شخصاً ليس لهم سوابق تعرُّض. كما أخذ الباحثون خزعات جَنْبَويَّة من الحالات المشتَبَه بها، وحددوا تركيز ألياف الأسبستوس المنقولة بالهواء في جميع المناطق. وشخَّصوا 88 حالة من ورم المتوسطة، 87 منها كانت ضمن المجموعة المعرَّضة. وتبين للباحثين أن اختطار ورم المتوسطة لدى المجموعة المعرَّضة بيئياً أعلى من المجموعتين الأخريين، كما أن الاختطار أعلى لدى الإناث منه لدى الذكور. وازداد انتشار ورم المتوسطة مع ازدياد التعرُّض التـراكمي للأسبستوس.
ABSTRACT: An epidemiological and environmental study was carried out in Shubra El-Kheima city, greater Cairo, of the exposure–response relationship between asbestos and malignant pleural mesothelioma. Radiological screening was done for 487 people occupationally exposed to asbestos, 2913 environmentally exposed to asbestos and a control group of 979 with no history of exposure. Pleural biopsy was done for suspicious cases. The airborne asbestos fibre concentrations were determined in all areas. There were 88 cases of mesothelioma diagnosed, 87 in the exposed group. The risk of mesothelioma was higher in the environmentally exposed group than other groups, and higher in females than males. The prevalence of mesothelioma increased with increased cumulative exposure to asbestos.
Exposition environnementale à l’amiante et relation exposition-réponse avec le mésothéliome
RÉSUMÉ: Une étude épidémiologique et environnementale a été réalisée à Shubra El-Kheima, dans la banlieue du Caire, sur la relation exposition-réponse entre l’amiante et le mésothéliome pleural malin. Un dépistage radiologique a été effectué sur 487 personnes exposées à l’amiante dans le milieu professionnel, 2 913 personnes exposées à l’amiante dans l’environnement et un groupe témoin de 979 personnes n’ayant jamais été exposées. Une biopsie pleurale a été réalisée sur les cas suspects. Les concentrations de fibre d’amiante dans l’air ont été établies dans toutes les zones. Quatre-vingt-huit cas de mésothéliome ont été diagnostiqués, dont 87 dans le groupe exposé. Le risque de mésothéliome était plus élevé dans le groupe soumis à une exposition environnementale que dans les autres groupes, et plus élevé chez les femmes que chez les hommes. La prévalence du mésothéliome augmentait en même temps que l’exposition cumulée à l’amiante .
1Department of Chest Diseases, Faculty of Medicine;
2Department of Medical Sciences, Institute of Environmental Studies and Research, Ain Shams University, Cairo, Egypt (Correspondence to M.T. Madkour:
3Department of Air Pollution, National Research Centre, Cairo, Egypt.
4Abbassia Chest Hospital, Cairo, Egypt. Received: 02/07/06; accepted: 04/12/06
EMHJ, 2009, 15(1): 25-38
Introduction
Malignant pleural mesothelioma (MPM) is associated with environmental and occupational exposure to asbestos [1]. During the last 4 decades, numerous studies on MPM have been conducted [2,3]. However, the natural history of this tumour remains ill-defined or scarcely known. Particular topics that deserve further investigation include the proportion of cases attributable to asbestos, the spectrum of the population at risk, the length of the latency period, the impact of mild exposure to asbestos and the role of cofactors in the development of the tumour [4].
Epidemiological studies have linked occupational exposure to asbestos with mesothelioma [5–10]. Cohort studies have found increased incidence of and mortality from mesothelioma among asbestos workers employed in mining [5], textile manufacturing [6], insulation [7] and asbestos cement factories [8]. Exposure has been assessed by a variety of methods, but all studies reach the conclusion that the rate of mesothelioma increases as the level of exposure increases [10]. Non-occupational or environmental exposure to asbestos is also associated with an increased risk of mesothelioma [11]. Exposure is experienced by individuals living with asbestos workers [12] and those living near asbestos mines and mills [13] or factories manufacturing asbestos products [14]. The development of the disease has a long latency, with cases arising more than 10 years after first exposure, and numbers continue to rise exponentially with time since first exposure [15].
Since other factors such as level of exposure also determine the risk of mesothelioma [16], it is important to analyse all these factors to disentangle their separate effects. The prevalence of mesothelioma has been increasing throughout the industrialized world [17]. The incidence is predicted to peak around 2020 [18]. This reflects industrial exposure to asbestos, which was common up to the 1980s, combined with a latent period between exposure to asbestos and development of mesothelioma averaging 30–40 years [17].
Data obtained from the information network of the General Organization for Industrialization in Egypt, showed that 14 asbestos factories were present in Egypt in the year 2004 [19]. These factories affect an area of approximately 5–7 km in radius, which explains the high incidence of mesothelioma in the neighbourhood of these factories. Workers employed since 1948 by the Egyptian asbestos company Sigwart at the mills in greater Cairo (El Maasara and Shubra El-Kheima) had an increased risk of mesothelioma, as did former residents of Shubra El-Kheima who were not directly employed in the milling of asbestos. In 2002, a high prevalence of pleuropulmonary disorders due to environmental asbestos exposure was reported in the immediate vicinity, 0.5–1.5 km, from an asbestos plant in south Cairo [20]. In Egypt, the ministerial council decided to ban asbestos imports in 2004 and the Sigwart plants were closed in November 2004. Therefore, the predicted incidence of mesothelioma in Egypt will reach its peak around 2040. However, no previous Egyptian study of environmental exposure to asbestos and the risk of mesothelioma has been able to utilize exposure levels to derive quantitative exposure–response relationships.
The aims of this study were: to evaluate the prevalence of MPM due to occupational and environmental (non-occupational) exposure to asbestos among persons who had worked in the asbestos manufacturing plant and in persons living in an area nearby the plant with potentially significant population exposure and to estimate the exposure–response relationship between environmental exposure to asbestos and MPM.
Methods
Location
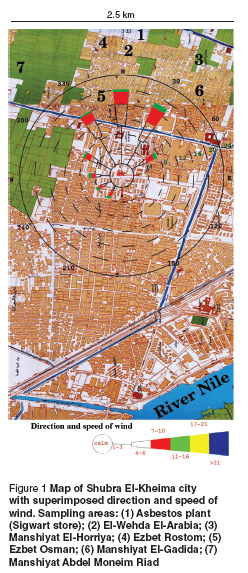
This epidemiological and environmental study was carried out in Shubra El-Kheima city, greater Cairo, to evaluate the prevalence of MPM. Shubra El-Kheima is an industrial city at the northern boundary of Cairo, just upwind from downtown Cairo. It has an area of about 30 km2. This city was considered the focal point of the highest environmental exposure to ambient asbestos fibres due to the operation of a large asbestos manufacturing plant (the Sigwart Company plant) (Figure 1). The Sigwart plant is an asbestos manufacturing plant using chrysotile asbestos. It was constructed in 1948 and its main products were asbestos cement pipes and reinforced concrete products. The study included 6 areas in the near vicinity of Sigwart plant, and these areas were mapped according to the distance from the asbestos plant and the airborne asbestos fibre concentrations.
Sample
The people working in the plant were designated the occupationally exposed group and the population living in the 6 areas near the plant were defined as the environmentally exposed group. Sampling areas of airborne asbestos fibres and the wind direction and speed are shown in Figure 1.
Environmentally exposed group
The 6 areas which were defined in the near vicinity of Sigwart plant included El-Wehda El-Arabia, Manshiyat El-Gadida, Manshiyat El-Horriya, Ezbet Osman, Manshiyat Abdel Moneim Riad and Ezbet Rostom. For the purpose of calculating population statistics, the 2000 census population data were obtained from the Egyptian Central Agency for Public Mobilization and Statistics. All sampling sites were located within 2 km of the asbestos plant. El-Wehda El-Arabia area includes 56 228 people and lies about 100 m walking distance of the asbestos plant. Manshiyat El-Gadida includes 20 000 people, Manshiyat El-Horriya includes 67 832 people, Ezbet Osman includes 25 000 people, Manshiyat Abdel Moneim Riad includes 128 215 people, finally, Ezbet Rostom includes 24 834 people. A total of 322 109 people are still living in these areas and are still exposed to asbestos.
Permission was taken from the health centres in these areas to do the study and in turn, the health visitors informed the population living in those areas to attend the centres to do a mass miniature radiography (MMR) scan. Considerable efforts were made to convince people of the importance of doing the MMR. Verbal consent was taken from all individuals included in the study.
The sample size was calculated as 3059 subjects using the Epi-Info program. Those who agreed to participate in the study were 2913 subjects who were chosen by cluster sampling, with a response rate of 95%. Details about occupation were taken, and subjects who were working in the plant or retired from the plant or had any history suggestive of occupational exposure to asbestos were excluded from the environmentally exposed group to avoid double registration.
Occupationally exposed group
Efforts were made to gain permission to do the study inside the asbestos plant and to convince the staff of the plant about the importance of having the MMR scan. The total number of staff working in the plant was about 543 people: 35 refused to participate in the study, 21 were lost during the study and the remaining 487 agreed to undergo the MMR. This group included workers dealing with asbestos manufacture in different processing sites (milling, manufacturing and cutting) and those working in the store, industrial shops, administration, asbestos waste site and the gate of the plant. The study was done during working hours in the presence of the whole workforce of the plant. All the staff inside the asbestos plant were exposed to the hazards of asbestos as there were contiguous rooms for all staff inside the different sections of the plant and clerks delivered circulars from the administration staff to the workers at their duty sites and there was supervision of work performance by administration staff.
Control group
An agricultural area at Banha city about 40 km from the plant was included in the study as a control area. The sample size of the population included in the study in that area was calculated as 1041 subjects using the Epi-Info program. Those who agreed to be included in the study were 979 people chosen by cluster sampling, a response rate of 94%. Verbal consent was taken before doing the MMR. None of these individuals had a history of occupational or environmental exposure to asbestos.
Data collection: clinical
Full history taking
All participants were interviewed. Occupational history was taken in full detail. The place of birth and residence were recorded in chronological order. The duration of environmental exposure to asbestos was determined. Full details were taken from the environmentally exposed group regarding their place of residence and period of residence in Shubra El-Kheima city. Care was also taken to exclude those with a history of occupational exposure to asbestos or retired people who were working in an asbestos plant. No history of migration was recorded because levels of mobility from one area to another in Egypt are very low and so the period from first residence in the area until the time of study was considered as the duration of environmental exposure.
Clinical examination
A thorough clinical examination was carried out for all subjects, with special emphasis on the respiratory system.
Mass miniature radiography
MMR was done for all studied groups. All the films were read by 2 qualified and experienced readers for diffuse parenchymatous disease and pleural diseases. We used MMR for the initial screening for the purpose of time and cost savings because of the large number of subjects in the study and also to avoid the health hazards of radiation to the community. This procedure is usually adopted by the Egyptian Ministry of Health surveys to detect cases with abnormal shadows and followed up with standard chest radiographs for confirmation.
Standard chest radiographs
Suspected cases with MMR abnormalities immediately underwent standard chest radiographs to confirm the presence of the abnormality.
High-resolution computerized scans
A high-resolution computerized scan (HRCT) of the chest was done for those persons with abnormal chest radiographs for better assessment and localization of the lesion.
Admission to Abbassia Chest Hospital
Subjects with abnormal MMR and abnormal HRCT were admitted to Abbassia Chest Hospital where pleural biopsy was done using thoracoscopy, limited thoracotomy or CT-guided biopsy.
Data collection: estimation of airborne asbestos fibres
The study was done in the period January 2003 to March 2004 inside the asbestos plant when it was functioning at full capacity and after the plant was closed in November 2004. So the screening of workers and other aspects of the study were done before closure of the plant.
Air sampling and analytical methods
Each air sample was collected on a membrane filter (Millipore AA, 25 mm diameter) mounted on an open filter holder. The air sampling was carried out for 8 hours at a flow rate of 10 L/min. All the samples were prepared by the method of the Occupational Safety and Health Agency (OSHA7400).
Filters were placed onto the surface of clean glass slides and were cleared using acetone triacetin reagent. A clean cover slip was gently lowered on the wedge at a slight angle to reduce bubble formation. The filter segment was outlined with a glass marking pen to aid in microscopic evaluation.
Measurements were carried out using light microscopy, phase contrast with a blue filter, adjustable field iris, 10 × eyepiece and 40 × phase objective (total magnification 400 ×). Walton Beckett graticule type with 100 μm diameter was used to count asbestos fibres (field area of Walton Beckett graticule is 0.00785 mm2). The slide was centred on the stage of the calibrated microscope under the objective lens, and the plane of the filter was focused on the microscope.
Fibres longer than 5 μm which lay entirely within the Walton Beckett graticule were counted. Counting started from the top of the filter wedge and progressed along a radial line of the outer edge, shifted up–down on the filter and continued in the reverse direction. The graticule field was chosen randomly. During 6 unit continuous scans a range of focal planes was examined by moving the 5 focus knobs to detect very fine fibres which may be embedded in the filter.
Calculations
Average count was calculated by dividing the total fibre count by the number of fields observed. Fibre density (E) (fibres/mm2) was defined as the average count (fibres/ field) divided by the field graticule area (AF) (0.00785 mm2):
Where F/nf = average fibre count/graticule field, B/nb = mean field blank count/graticule field.
The concentration (C) (fibres/cm3) of fibres in the air volume (V) (L) using the effective collection area of the filter (AC) (385 mm2) was calculated as follows:
V = L/min × times = 3.1 × 30 = 93 L.
Sampling sites
Inside the asbestos plant: 45 air samples were taken from all department, the milling site (8 samples), the manufacturing machine (7 samples), the cutting site (6 samples), asbestos waste site (5 samples), industrial shops (5 samples), the gate (4 samples), the administration office (5 samples), and the store (5 samples). The samples were collected in the working environment dealing with the manufacture of the asbestos pipes during different processes. The air samples were collected at a height of 1.5 m (breathing zone) above the ground level between 09:00 and 17:00 hours.
Outside the asbestos plant: air samples were collected at 6 different areas (5 samples each) in Shubra El-Kheima city around the plant in a radius of 2.5 km.
Agricultural area: air samples were collected from an agricultural area at Banha city, about 40 km from the plant.
Methods of estimation of individual exposure levels
Intensity of exposure was calculated according to airborne asbestos fibre concentration (f/mL). Duration of exposure was determined for all subjects. Duration of exposure was then combined with intensity of exposure to give a measure of cumulative exposure for each person in fibres per millilitre years (f/mL-years). Latency was estimated from the year of first exposure until the onset of symptoms.
Statistical methods
Data were analysed using SPSS, version 9. Statistical analysis was carried out using Student t-test, Mann–Whitney test, chi-squared test and Fisher test. Relative risks (RR) and 95% confidence intervals (95% CI) were calculated to determine the risk of MPM in exposed subjects.
Results
The demographic and exposure characteristics of asbestos-exposed subjects are shown in Table 1. There was a highly statistically significant difference between the environmentally and occupationally exposed groups as regards age, sex, duration of exposure (i.e. length of time resident in the area or employed in the factory) and prevalence of MPM cases (P < 0.001).
The classification of pleuropulmonary disorders in all study groups is shown in Table 2. There were 88 cases of MPM diagnosed, 87 in the exposed groups and 1 in the control group. MPM was more prevalent in the environmentally exposed group (83/2913, 2.8%) than the occupationally exposed group (4/487, 0.8%) and control group (1/979, 0.1%) (Table 3).
The mean age of patients with MPM was 51.3 [standard deviation (SD) 8.08] years; 54.1 (SD 8.45) years (range 39–70 years) for males and 49.5 (SD 7.39) years (range 35–60 years) for females.
The exposure characteristics of subjects positive and negative for MPM are shown in Table 4. Exposure to asbestos was significantly higher among patients with MPM compared to non-MPM subjects.
The prevalence of MPM among the environmentally exposed group in different areas is shown in Table 5. There was a statistically significantly higher prevalence of MPM cases in El-Wehda El-Arabia area (4.5%) compared to Ezbet Rostom area which had the lowest prevalence (1.2%) (χ2 = 12.75, P = 0.03).
The risk of developing MPM was 26 times greater among the occupationally and environmentally exposed groups compared to the non-exposed group (Table 6).
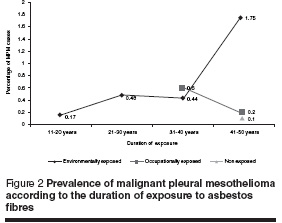
The duration of exposure to asbestos in cases of MPM in all studied groups is shown in Table 7. There was a significantly number of MPM cases in patients with environmental asbestos exposure of more than 40 years when compared to those with a shorter duration of exposure (P < 0.001). Figure 2 shows that the percentage of MPM cases increased with longer duration of exposure to asbestos fibres.
Fibre concentrations in the different sites inside the asbestos plant are shown in Table 8. There was a highly significant difference between fibre concentrations at the asbestos waste site versus other sites and versus the control area (P < 0.001). Also there was a highly significant difference between fibre concentrations at asbestos manufacturing sites (milling, cutting and industrial shops) versus the control area (P < 0.001). Airborne asbestos fibre concentrations in the surrounding areas outside the asbestos plant and number of MPM cases are shown in Table 9. The overall mean asbestos fibre concentration in the exposure areas was 0.38 (SD 0.87) f/mL. The number of cases of MPM was higher in El-Wehda El-Arabia area (39 cases), where the concentration of airborne asbestos fibres was higher (2.16 f/mL) compared with other areas (P < 0.01).
The distribution of various indices of estimated exposure for MPM and non-MPM cases is shown in Table 10. Nearly 50% of patients with MPM had cumulative exposure > 20 f/mL-years.
Discussion
In this study 88 cases of MPM were diagnosed, all except 1 in the asbestos-exposed groups. The finding of 1 mesothelioma case in the non-exposed group can be explained by Hillerdal in 1999 who discussed the evidence that the tumour can occur even in the complete absence of asbestos, i.e. a spontaneously occurring tumour [2].
The mean age of MPM cases was 54.1 years. These results are in agreement with a study which reported that malignant mesotheliomas of the pleura may occur over a wide age range, but are most commonly observed in adults over the age of 50 years [21].
Our study showed a significant difference in age in all exposed groups including MPM cases between the occupationally exposed and environmentally exposed groups. This might be because workers inside the asbestos plant were exposed to higher concentrations of asbestos fibres than the environmentally exposed group, so the mesothelioma might occur at an earlier age than the environmentally exposed group or there might be retirement at an earlier age in addition to death from MPM at an earlier age in the workers.
The female predominance of MPM cases (61.4% versus 38.6%) in our study is in agreement with another study which found that out of 27 cases with malignant pleural mesothelioma, 18 cases were females (66.7%) and 9 cases were males (33.3%) [10].
In Egypt, one study found that the dust concentration in the environment of Sigwart factory was 3–20 f/mL [22]. Our results showed a much lower mean concentration of 0.59 f/mL, presumably due to improvements in the technology of asbestos manufacturing and the use of closed systems along with the application of filters, frequent cleaning, suction systems and protective masks for the workers which led to a decrease in the concentration of the airborne asbestos fibres.
Environmental assessment of airborne asbestos fibre concentrations showed that the highest mean concentration (2.16 f/mL) was in the El-Wehda El-Arabia area which lies within 100 m of the asbestos plant and where the wind direction is towards that area. Fibre concentrations start to decrease further away from the plant. The overall mean asbestos fibre concentration in the exposure areas was 0.38 f/mL. This was in contrast with the findings of another study reporting a concentration of 7–13 f/mL in an area 0.5–1.5 km from the asbestos plant [20]. Therefore, it seems that exposure to asbestos fibres is not only limited to workers in factories but to those living in areas with environmental pollution from asbestos fibres.
In a study from Turkey the authors measured the airborne fibre concentration in a village environmentally exposed to asbestos, where they found that the mean concentration was 0.012 f/mL [23], a figure lower than ours. Their study and others [10,24] showed a higher rate of MPM cases among those with higher intensity and cumulative exposure to asbestos. This was also confirmed in our study which showed a positive correlation between the asbestos fibre concentration and the number of MPM cases in all studied areas. It should be stressed that the high number of women detected with mesothelioma is due to the long duration of residency near the asbestos plant with high permissible concentrations of asbestos.
The current study showed that MPM was more prevalent in the environmentally exposed group (2.8%) than in the occupationally exposed group (0.8%) reflecting the impact of environmental air pollution with asbestos on the prevalence of mesothelioma. This is in agreement with a South African report which showed that one-third of the mesothelioma cases were exposed as residents living near asbestos mines and mills [25].
Comparing the prevalence of MPM among the environmentally exposed group in the different areas, our study showed that MPM was more prevalent in El Wehda El Arabia (4.5%), the nearest area near to the asbestos plant, than other areas further away. This agrees with the results of other studies which detected excess cases in the immediate neighbourhood of factories that processed asbestos, mainly from the same South African mines [26–28].
As regards monitoring of airborne asbestos fibre concentration, our study showed that the mean occupational airborne asbestos concentrations (0.59 f/mL) were significantly higher than the environmental concentrations (0.38 f/ mL). Similar results have been shown in previous reports on airborne asbestos concentrations in Europe and the United States of America [29].
Conclusion
This study provides additional quantitative information on the relative risks of pleural mesothelioma in subjects environmentally exposed to asbestos. Of special interest is the 26-fold excess risk of pleural mesothelioma due to environmental exposure. The central role of latency and of cumulative exposure in determining the risk of mesothelioma is of special importance. The present study has an important message. The mesothelioma epidemic will affect areas without major industrial exposure to asbestos. Though the importation of raw asbestos into Egypt was banned at the end of 2004, the threat of developing mesothelioma will remain for a considerable period of time due to the latency period. Therefore, a high index of suspicion is needed for early detection of mesothelioma in persons with environmental exposure to asbestos.
Successful interventions have already been made by the government of Egypt to prevent further exposure to asbestos by placing a ban on its use. The focus now shifts to small informal workshops which use asbestos with a high risk of environmental exposure. Despite technical difficulties, the process of replacing asbestos with safer materials, especially in small informal workshops, is essential to prevent further release of asbestos into the environment. Industries with harmful environmental and health impacts are heavily regulated in the developed countries and these industries are migrating to the developing world. Therefore great caution is needed, especially in the construction industry with the growing use of asbestos-based cement products.
References
- Selcuk ZT et al. Malignant pleural mesothelioma due to environmental mineral fiber exposure in Turkey. Analysis of 135 cases. Chest, 1992, 102(3):790–6.
- Hillerdal G. Mesothelioma: cases associated with non-occupational and low dose exposure. Occupational environmental medicine, 1999, 56:505–13.
- Giarelli L, Bianchi C. Geography of mesothelioma: expected findings and paradoxes. European journal of oncology, 2000, 5(Suppl. 2):77–81.
- Bianchi C et al. Asbestos exposure in malignant mesothelioma of the pleura: A survey of 557 cases. Industrial health, 2001, 39:161–7.
- McDonald JC et al. The 1891–1920 birth cohort of Quebec chrysotile miners and millers. Mortality 1976–88. British journal of industrial medicine, 1993, 50:1073–81.
- Yano E et al. Cancer mortality among workers exposed to amphibole free chrysotile asbestos. American journal of epidemiology, 2001, 154:538–43.
- Selikoff IJ, Hammond EC, Sediman H. Mortality experience of insulation workers in the United States and Canada, 1943–1976. Annals of the New York Academy of Sciences, 1979, 330:91–116.
- Ulvestad B et al. Cancer incidence among workers in the asbestos cement producing industry in Norway. Scandinavian journal of work environment and health, 2002, 28(6):411–7.
- Dement JM, Brown DP, Okun A. Follow up study of chrysotile textile workers: cohort mortality and case–control analyses. American journal of industrial medicine, 1994, 26:431–47.
- Hansen J et al. Environmental exposure to crocidolite and mesothelioma exposure–response relationships. American journal of respiratory critical care medicine, 1998, 157:69–75.
- 11. Joubert LH, Sediman H, Selikoff IJ. Mortality experience of family contacts of asbestos factory workers. Annals of the New York Academy of Sciences, 1991, 643:416–8.
- Dodoli D et al. Environmental household exposure and occurrence of pleural mesothelioma. American journal of industrial medicine, 1992, 21:681–7.
- Reid GD et al. Mortality of an asbestos-exposed birth cohort: a pilot study. South African medical journal, 1990, 78:684–6.
- Hammond EC, Garfinkle L, Selikoff IT. Mortality experience of residents in the neighbourhood of an asbestos factory. Annals of the New York Academy of Sciences, 1979, 330:417–22.
- Deklerk NH, Armstrong BK. Cancer mortality in relation to crocidolite at Wittenoom Gorge in Western Australia. British journal of industrial medicine, 1989, 46:529–36.
- Hauptmann M et al. The exposure-time response relationship between occupational asbestos exposure and lung cancer in two German case–control studies. American journal of industrial medicine, 2002, 41:89–97.
- Britton M. The epidemiology of mesothelioma. Seminars in oncology, 2002, 29:18–25.
- White C. Annual deaths from mesothelioma in Britain to reach 2000 by 2010. British medical journal, 2003, 325:1417.
- Kazan-Allen L. Asbestos: the environmental hazard. In: Proceedings of the International Conference Asbestos Risk Reduction and Measurement of Asbestos Fibre Concentration, Cracow, Poland, September 28–29, 2006. Krakow, Faculty of Materials Science and Ceramics, AGH University of Science and Technology (www.ceramika.agh.edu.pl/azbest06/2.pdf, accessed 11 August 2008).
- Tag-El-Din MA et al. Environmental airborne asbestos pollution and pleuropulmonary disorders in South Cairo. Egyptian journal of chest diseases and tuberculosis, 2002, 51(2):160–6.
- Sherad JD et al. Pneumothorax and malignant mesothelioma in patients over the age of 40. Thorax, 1991, 46:584.
- El-Shaer ARA et al. Epidemiological study of asbestos-related diseases among Egyptian workers exposed to asbestos [MD thesis]. Cairo, Department of Industrial Medicine and Occupational Health, Al-Azhar University, 1989.
- Metintas S et al. Malignant mesothelioma due to environmental exposure to asbestos. Follow up of a Turkish cohort living in a rural area. Chest, 2002, 122:2224–9.
- Browne K. Asbestos-related mesothelioma, factors discriminating between pleural and peritoneal sites. British journal of industrial medicine, 1986, 40:145–9.
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the north western Cape Province. British journal of industrial medicine, 1960, 17:260–71.
- Selden A et al. Exposure to tremolite asbestos and respiratory health in Swedish dolomite workers. Occupational environmental medicine, 2001, 58:670–7.
- Hain E et al. Katamnestische Untersuchungen zur Genese des Mesothelioms. Bericht uber 150 Falle aus dem Hamburger Raum [Retrospective study of 150 cases of mesothelioma in Hamburg area]. Internationales Archiv für Arbeitsmedizin, 1974, 33:15–37.
- Magnani C et al. Malignant pleural mesothelioma and non-occupational exposure to asbestos in Castale Monferrato, Italy. Occupational and environmental medicine, 1995, 52:362–7.
- Burdett GJ et al. Mean concentrations of airborne asbestos in the non-occupational environment. Annals of occupational hygiene, 1994, 28:31–8.








