Y.E. Himeidan1 and E.A. El Rayah2
دور بعض العوامل البيئية في تكاثر الأنوفيلة العربية في مدينة حلفا الجديدة، بشرق السودان
يوسف الصافي حميدان، الأمين الريح
الخلاصـة: أجريت دراسـة حول النشـاط التكاثري للأنوفيلـة العربيـة في مـا بين آذار/مـارس 1999 وحزيران/ يونيو 2000 في مدينة حلفا الجديدة. ولقد تم فيها جمع 3642 يرقة أنوفيلية، كانت 3633 منها (99.75%) تنتمي للأنوفيلة العربية: 82.49%، منها جمعت من برك المياه الضحلة المعرَّضة للشمس والناجمة عن التسريبات من المواسير، و11.56% منها من مياه الأمطار، و3.08% من قنوات الري، و2.88% منها من البِرَك الاصطناعية. وبلغ متوسط الكثافة الإجمالية 24.34 يرقة/10 غَرْفات، بواقع 40.73 في موسم الأمطار، و 30.45 في موسم الري، و13.10 في موسم الجفاف. أما مياه زراعة المحاصيل فقد زادت الرطوبة النسبية (0.013=P)، مما أدى إلى زيادة النشاط التكاثري للأنوفيلة العربية (0.005P<). p="">
ABSTRACT: Anopheles arabiensis breeding was studied during March 1999–June 2000 in New Halfa town. Of 3642 anopheline larvae collected, 3633 (99.75%) were A. arabiensis: 82.49%, 11.56%, 3.08% and 2.88% of the larvae were collected from shallow sunlit pools resulting from pipes leakages, rain pools, irrigation canals and man-made pools respectively. The overall mean density was 24.34 larvae/10 dips: 40.73 during the rainy season, 30.45 during irrigation and 13.10 in the dry season. Water for crop cultivation increased the relative humidity (P = 0.013) and both factors enhanced A. arabiensis breeding (P < 0.005). There was no significant difference between the rainy and irrigation seasons. A. arabiensis breeding in this area has become perennial as a result of crop irrigation.
Rôle de certains facteurs environnementaux sur l’activité de reproduction d’Anopheles arabiensis à New Halfa (Soudan oriental)
RÉSUMÉ: La reproduction d’Anopheles arabiensis a été étudiée entre mars 1999 et juin 2000 à New Halfa. Sur 3642 larves d’anophèle récoltées, 3633 (99,75 %) étaient des A. arabiensis : 82,49 %, 11,56 %, 3,08 % et 2,88 % des larves ont été prélevées dans des points d’eau peu profonds et ensoleillés résultant respectivement de fuites de canalisations, de pluies, de canaux d’irrigation et de plans d’eau artificiels. La densité moyenne globale était de 24,34 larves/10 prélèvements : 40,73 pendant la saison des pluies, 30,45 pendant la saison d’irrigation et 13,10 pendant la saison sèche. L’eau destinée aux cultures augmentait l’humidité relative (p = 0,013) et ces deux facteurs favorisaient la reproduction d’A. arabiensis (p < 0,005). Il n’existait pas de différence significative entre la saison des pluies et la saison d’irrigation. La reproduction d’A. arabiensis dans cette région dure désormais toute l’année du fait de l’irrigation des cultures.
1Entomology Unit, Faculty of Agriculture and Natural Resources, University of Kassala, New Halfa, Sudan (Correspondence to Y.El-S. Himeidan:
2Department of Zoology, Faculty of Science, University of Khartoum, Khartoum, Sudan.
Received: 29/08/05; accepted: 04/01/06
EMHJ, 2008, 14(5) 252-259
Introduction
Malaria is a major public health problem and more than 1 million people die every year from the direct causes of the disease with almost 90% currently concentrated in sub-Saharan Africa [1]. The control of malaria through chemotherapy has been confronted by the spread of Plasmodium falciparum resistance to antimalarial drugs [2,3]. So, efforts to reduce vector abundance are urgently needed to supplement the drug-based curative approach in the malaria control interventions. Approximately 90% of the malaria burden is related to environmental factors [4]. The establishment and operation of water resource development projects is an important aspect of such environmental factors, since changes in the transmission pattern of the disease following irrigation development have been reported [5]. A major study has documented increases in malaria transmission as a result of irrigation and agricultural development [6]. This is more obvious in areas with unstable malaria transmission than in areas with stable transmission where the introduction of irrigation schemes has no or little influence on disease prevalence [5–9]. However, the reasons for this protection are attributed partially to good access to effective antimalarial drugs and personal protection, e.g. sleeping under insecticide-treated bed nets [5]. Thus, malaria intervention methods should be adapted to local environmental conditions as there is no single approach that is applicable to all situations.
In areas with high levels of antimalarial drug resistance, vector control is the most practical method for reducing malaria transmission [10]. A detailed knowledge of the ecology of the local malaria vector is essential in the design of effective methods for controlling the disease by vector control measures. In Sudan, very few data are available about the ecology of the principal vector, Anopheles arabiensis, particularly in agricultural areas where irrigation has significantly increased the vector density [11–13]. The present study was conducted to elucidate the factors affecting the breeding activity of the vector A. arabiensis in New Halfa town, the site of the second largest irrigation scheme in Sudan.
Methods
Study area
The study was carried out in New Halfa town (altitude 450 m, 15o 19´N & 35o 36´E), which was previously described by Himeidan et al. [12]. It is located in the middle of the irrigated area with a climate characterized by a short rainy season (July–October) followed by a cool dry season with extensive irrigation activity (November–February) and then a hot dry season (March–June). Monthly mean maximum and minimum temperature, relative humidity and the total annual rainfall during the study period were 38.7 oC, 22.1 oC, 41.7% and 411.4 mm respectively. The hottest months of the year are April (22.8–42 oC) and May (27.2–42.8 oC) immediately prior to the rainy season. The topography of the area is nearly flat (slope is less than 2%) with many bit holds formed as a result of high content of montmoillonitic type of clay (50%–60%). This type of soil shrinks when it dries up and expands when it becomes wet [14]. This seasonal movement of soil results in a considerable leakage of the drinking water pipes every year.
P. falciparum is the predominant malaria parasite species in the area with a high level of resistance to antimalarial drugs [3]; A. arabiensis is the principal malaria vector [12]. Mosquito control measures are limited to indoor residual spraying during the rainy season.
Sampling
Immature stages of A. arabiensis were sampled monthly at New Halfa town from March 1999 to June 2000. The sampling was carried out from 13:00 to15:00 using a standard dipper at all positive breeding sites that had been found in the area. The number of different larval instars was counted separately in 10 dippers. All collected anopheline larvae were transferred to the laboratory and their numbers in different instars were recorded following the standard method [15]. All samples were reared until adulthood to facilitate species identification by morphological characteristics [16].
Data on climate variables and water intended for crop irrigation (m3/month) during the study period were obtained from the meteorological and irrigation stations at New Halfa town. Irrigation water was measured as the monthly amount of water available for crop irrigation in the New Halfa agriculture scheme. The amount of water fluctuates depending on the yearly amount of water available in Khashm El Girba dam and on the actual monthly amount of water released from the dam to irrigate the crops cultivated in a specific time and area.
Statistical analysis
Data were analysed using SPSS, version 12. ANOVA (one-way analysis of variance) was used to compare the differences in the mean density of larvae at different seasons. Linear regression analysis was carried out to correlate the density of immature stages with monthly climatic variables and water intended for crop irrigation.
Results
Out of 3894 anopheline larvae collected, 3642 (93.53%) were successfully reared to adulthood. A. arabiensis was the predominant species in the area and accounted for 99.75% (3633/3642) of the adults that emerged, while A. pharoensis constituted only 0.25%. A number of Culex species were occasionally collected from the same pools.
The majority (82.49%) of the larvae were collected from the shallow sunlit pools resulting from the broken water pipes. Other breeding sites included: rain pools (11.56%), puddles of irrigation canals (stretches of water extending about 500 m from leakages of irrigation canals) (3.08%) and man-made pools (2.88%). The irrigation pools were seen occasionally during March and were limited to the edge of the town, so the few larvae collected do not reflect the ecological importance of this habitat in the area. The proportions of all A. arabiensis first instar larvae recorded in these breeding habitats were 89.2%, 4.7%, 1.2% and 4.9% respectively. Figure 1 shows the distribution of A. arabiensis by season and breeding habitat.
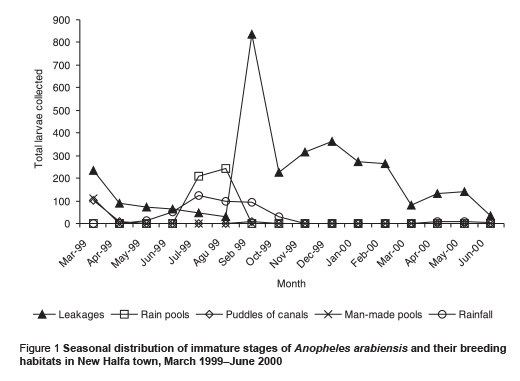
Breeding activity of A. arabiensis was found throughout the study period, even during the 2 dry seasons. The mean number of immature larvae/10 dips collected during the study period was 24.34 (95% confidence interval: 13.5–35.1) larvae/10 dips. There was a significant difference in the densities of immature stages between the dry and rainy seasons (P = 0.022). The highest number of immature stages (mean = 40.73 larvae/10 dips) were collected during the rainy season; the peak of density lag was approximately 1 month after the peak in rainfall. During the cool dry (irrigation) season (November–February) immature stages of A. arabiensis were present in relatively high densities (mean = 30.45 larvae/10 dips). For the 2 dry seasons mean larvae density was low (13.10 larvae/10 dips) (Table 1 and Figure 1).
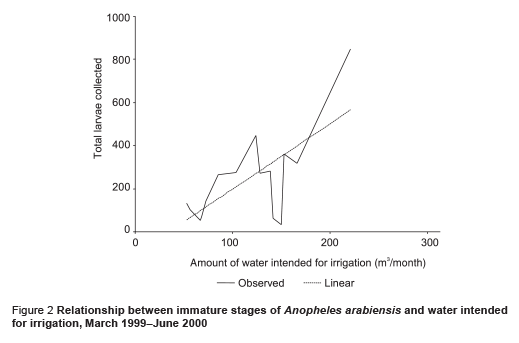
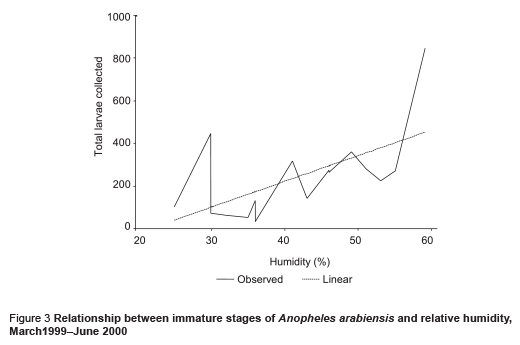
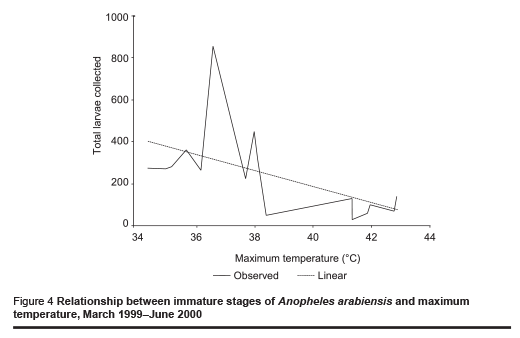
The abundance of immature stages of A. arabiensis showed a statistically significant positive relationship with water intended for crop irrigation (m3/month) (correlation coefficient ρ = 0.70, P = 0.002) and the rise in the relative humidity (correlation coefficient ρ = 0.65, P = 0.005) (Figures 2 and 3). A significant negative correlation was found with maximum temperature (correlation coefficient ρ = –0.571, P = 0.013) (Figure 4). Increase in water intended for crop irrigation was significantly associated with an increase in relative humidity (correlation coefficient ρ = 0.573, P = 0.013). These were the 2 climatic variables that enhanced the larval density.
Discussion
The present ecological surveys were conducted on malaria vectors in New Halfa town an irrigated area in the eastern Sudan. The study confirmed that A. arabiensis is the predominant malaria vector in the area. This is in accordance with previous observations in the same area and different parts of northern Sudan [11,12,17]. The breeding of mosquitoes was reported throughout the year, even during the hottest months (April and May) (during the 2 dry seasons) (Table 1 and Figure 1). Similar observations have been reported in irrigated areas in the Nile valley in central and northern Sudan [13,18]. This differs from the situation in the nearby area in Gadaref state where A. arabiensis disappears in the hot dry season (March–May) [11]. In contrast, in a desert area about 20 km south-east of Omdurman, it has been reported that adult A. arabiensis females survive the long hot dry season, probably in a state of lowered physiological activity described as incomplete aestivation to adapt to the harsh summer conditions [19]. In an alternative environment, in dry savannah, the breeding activity of A. arabiensis is entirely limited to watery microenvironments and, after a sharp contraction in effective population size during the dry season, local populations might rebound each year [20]. Even under the highest temperature conditions, a low level of vector breeding continued in our study area (Figure 4).
We found that the introduction of a permanent irrigation system in the study area enhanced the 2 essential ecological factors for larval development: relative humidity and breeding habitats. It is well known that temperature and humidity affect adult and immature stages of A. arabiensis adversely [21]. The impact of irrigation was more pronounced during the cool dry season when the main crops cotton and wheat are cultivated. During this period, a minor peak in the population density was observed but the difference was not statistically significant in comparison with the main peak in the rainy season (P = 0.417). Water intended for crop irrigation was significantly associated with an increase in the density of immature stages. Hence, the second peak of immature stages could be attributed to the period of growing crops. However, the lack of proper maintenance of the irrigation canals led to water logging and creation of ideal breeding sites for the principal vector A. arabiensis, a species that prefers to breed in open sunlit pools [8,22,23]. This was actually observed during March 1999 (Figure 1). Therefore, the breeding activity of the vector appears to be becoming perennial instead of seasonal as a result of irrigation of crops.
During the dry season, the continuous breeding activity of the vector A. arabiensis was reported mainly in the shallow sunlit pools resulting from leakages of water pipes (Figure 1). These breaks were found to be the most important attractive breeding habitats for the vector to lay its eggs. According to the water supplies corporation of the town, the seasonal movement of soil could explain these breaks of pipes, which is associated with climatic conditions, i.e. temperature affects the clay soil expansion and contraction and may cause pipes to leak [14]. However, in the rainy season, larvae were observed to increase rapidly after the onset of rain in a similar way to other African countries that are characterized by dry savannah [11,20,21]. The densities during this season rose 1 month after the peak in rainfall. Hamad et al. attributed this phenomenon in eastern Sudan to the characteristics of the rainfall, where breeding was possible in small pools [11]. However, frequent heavy rainfall may kill or flush away the immature stages, although, Robert et al. [24] have reported that the shocks due to raindrops are not noticeable mortality factors for anopheline larvae. Evidently mosquitoes do breed during the short rainy season, but they may be subject to death as a result of natural or physical factors. Nonetheless, in our study area the mosquito population increased rapidly until the end of the rainy season, when the shortage of breeding sites and the change in climatic conditions led to a decrease in population densities.
Clearly, the breeding activity of A. arabiensis in this area is governed by both rainfall and periods of crop irrigation, and has now become perennial rather than seasonal as a result of the latter, and at no time during the year did the temperature go above or fall below the optimum range of the mosquito [25].
Acknowledgements
The authors wish to thank Dr Dia Eldeen Elnaem, Dr Ishag Adam and Dr Mustafa Idris Elbashir for their valuable comments on the manuscript.
References
- The World Health Report 2004 – Changing history. Geneva, World Health Organization, 2004.
- Marsh K. Malaria disaster in Africa. Lancet, 1998, 352:924.
- Adam I et al. Efficacies of chloroquine, sulfadoxine–pyrimethamine and quinine in the treatment of uncomplicated, Plasmodium falciparum malaria in eastern Sudan. Annals tropical medicine and parasitololgy, 2004, 98:661–6.
- Health and environment in sustainable development. Five years after the Earth Summit. Geneva, World Health Organization, 1997.
- Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Medical and veterinary entomology, 2001, 15:1–11.
- Ghebreyesus TA et al. Incidence of malaria among children living near dams in northern Ethiopia: community based incidence survey. British medical journal, 1999, 319:663–6.
- Boudin C et al. Epidemiology of Plasmodium falciparum in a rice field and a savannah area in Borkina Faso. Comparative study on the acquired immunoprotection in native populations. Acta tropica, 1992, 51:103–11.
- Mutero CM et al. Water management for controlling the breeding of Anopheles mosquitoes in rice irrigation schemes in Kenya. Acta tropica, 2000, 76:253–63.
- Klinkenberg E et al. The phenology of malaria mosquitoes in irrigated rice fields in Mali. Acta tropica, 2003, 85:71–82.
- Global malaria control. WHO Malaria Unit. Bulletin of the World Health Organization, 1993, 71:281–4.
- Hamad AA et al. Marked seasonality of malaria transmission in two rural sites in eastern Sudan. Acta tropica, 2002, 83:71–82.
- Himiedan YE et al. Anopheles arabiensis: abundance and insecticide resistance in an irrigated area of eastern Sudan. Eastern Mediterranean health journal, 2004, 10(1/2):167–74.
- El Gaddal AA et al. Malaria control in the Gezira-Managil Irrigated Scheme of the Sudan. Journal of tropical medicine and hygiene, 1985, 88:153–9.
- Ochtman LHJ. Soil survey report of Khashm El-Girba Scheme. Wad Medani, Soil Survey Division, Gezira Research Station, 1964.
- Service MW. Mosquito ecology. Field sampling methods. London, Applied Science Publisher, 1976.
- Gillies MT, DeMeillon B. The Anopheline of Africa south of the Sahara (Ethiopian Zoogeographical Region), 2nd ed. Johannesburg, South Africa Institute Medical Research, 1968 (No. 54).
- Petrarca V et al. Cytogenetics of the Anopheles gambiae complex in Sudan, with special reference to An. arabiensis: relationships with East and West Africa populations. Medical and veterinary entomology, 2000, 14:149–64.
- Dukeen MY, Omer SM. Ecology of the malaria vector Anopheles arabiensis Patton (Diptera:Culicidae) by the Nile in northern Sudan. Bulletin of entomological research, 1986, 76:451–67.
- Omer SM, Cloudsely-Thompson JL. Survival of female Giles through the 9 month dry season in Sudan. Bulletin of the World Health Organization, 1970, 42:319–30.
- Taylor CE et al. Effective population size and persistence of Anopheles arabiensis during the dry season in West Africa. Medical and veterinary entomology, 1993, 7:351–7.
- Molineaux L et al. The epidemiology of malaria and its measurement. In: Wernsdorfer WH, McGregor I, eds. Malaria: principles and practice of malariology. Edinburgh, Churchill Livingstone, 1988:999–1090.
- Mwangi RW, Mukiama TK. Irrigation scheme or mosquito hazard: a case study in Mwea Irrigated Scheme. Hydrobiologia, 1992, 232:19–22.
- White GB. The Anopheles gambiae complex and malaria transmission around Kisumu, Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1972, 66:572–81.
- Robert V et al. Rainfall is not a direct mortality factor for Anopheline larvae. Parasite, 1999, 6:195–6.
- MacDonald G. The epidemiology and control of malaria. London, Oxford University Press, 1957.








