Zafar Mirza1
1Regional Adviser, Essential Medicines & Pharmaceutical Policies, World Health Organization Regional Office for the Eastern Mediterranean, Cairo, Egypt (Correspondence to Zafar Mirza:
EMHJ, 2008, 14(Supplement): S74-S81
Introduction
The development of the first Model List of Essential Drugs2 [1] and the Declaration of Alma Ata [2], which advocated the adoption of primary health care (PHC), were both important milestones in the history of public health. Some elements of both were already in practice in some countries before the late 1970s when the World Health Organization (WHO) adopted and promoted them. They are dependent on each other for their success and they continue to bolster each other. Thirty years of the Alma Ata Declaration is also a history of essential medicines – the concept and its application. It is time to celebrate, reflect and revisit these time-tested concepts in order to face the future challenges for the organization and provision of effective health care services and health systems.
This article first presents the conceptual need for and rationale of essential medicines, from its beginnings until now. Essential medicines, as an integral component of the PHC philosophy and system, are explained and developments in the essential medicines approach over the past 30 years are traced. The situation with regard to essential medicines in the East Mediterranean Region of the World Health Organization is briefly presented. Finally the existing and future challenges to PHC and essential medicines are defined and their relevance in the changing times and contexts of the 21st century is established.
Why essential medicines?
The rationale for essential medicines can be explained from both the demand and supply perspectives.
From the demand perspective, in a typical low or low-to-middle income country, people from low socioeconomic strata continue to suffer and die from preventable and curable diseases. The most vulnerable – children, women, the elderly – suffer the most. Their medicine needs are served only marginally by the public sector health facilities; they are generally not protected socially for their health care needs. Thus the overwhelming majority has no choice but to take from their pitifully shallow pockets to try and buy their medicines from the packed, thriving and poorly regulated private retail pharmacies. But many of the poor are not able to buy their treatments from the private sector even if they are willing. The rising number of poor in industrialized countries are now also suffering similarly. This is a disturbing paradox of modern health care systems.
From the supply perspective, discovery, development and delivery of medicines – an innovation cycle [3] – is primarily market driven. It is not sensitive to public health needs, especially those in developing countries. This wheel of innovation inherently ignores the diseases of poverty and the poor. Motivated by return-on-investment, the pharmaceutical industry and private pharmacies fill the shelves with products that can bring more money and focus on those consumers who can buy. Long supply chains are established that involve innumerable middle-men who make a profit at each step. Science and public health needs are compromised in this enterprise, and what has been created is a proverbial “therapeutic jungle” [4] and a complete lack of essential treatments.
These demand and supply situations in the pharmaceutical sector are neither new nor diminishing. Instead, they are inherent and ever growing.
Before WHO became involved in the essential drug concept, governments had been concerned about the situation for years and some had started to introduce innovative polices and management tools to meet the therapeutic needs of their populations by providing them with the most needed medicines. Among developing countries that had started some sort of selection process leading to national drug lists were Sri Lanka, Costa Rica, Peru, Cuba, Egypt, Papua New Guinea, Mozambique and Tanzania. In the more developed world, Scandinavian countries, Canada and Australia had such selection processes in place.
Inspired by these national initiatives and concerned about the pharmaceutical situation, especially in poor settings, WHO gradually became involved in these issues. In May 1975, Dr Halfdan Mahler, Director-General of WHO at the time, strongly advocated before the Member States at the World Health Assembly for the development of national pharmaceutical policies based on the affordability, quality and availability of drugs [5]. A resolution was passed which urged the Secretariat of WHO to help Member States to formulate national pharmaceutical policies that meet the actual health needs of the people [6]. Rapidly, the concepts of “essential drugs” and “national drug policy” entered the vocabulary of global public health [7]. After the compilation of national practices based on lists of basic drugs in 1976, the first meeting of the Expert Committee on Selection of Essential Drugs was held [8] and in 1977 WHO adopted the first Model List of Essential Drugs [1]. Since then it has been reviewed and updated every 2 years and the current WHO Model List is its 15th edition. Whereas in 1977 only around a dozen countries had what could be considered a national essential medicines list (NEML), today 4 out of 5 countries have one, i.e. 156 countries out of 193 Member States of WHO.
The basic idea behind the essential medicines concept is that while there are many medicines registered and available on the market, it is important to be selective, bearing in mind the medical needs of the majority of the population, and to ensure the efficacy, safety and cost– effectiveness of the medicines. By following this strategy most needed medicines can be supplied to a maximum number of people. The approach is fair, efficient and above all based on common sense. This is also a way of overcoming imperfections and failures of the pharmaceutical market. The concept is not only applicable in the public sector where it is most used but also in the private sector, especially in health insurance systems. It contributes to achieving health objectives and is based on sound economics and ethics.
Essential medicines and PHC
Chronologically, the development of the first model list of essential medicines in 1977 coincided with the Health for All strategy [9], both of which preceded the Alma Ata Declaration that brought PHC into being in 1978. The PHC advocated in the Declaration of Alma Ata was based on 8 fundamental elements, one of which was the “provision of essential drugs”.
When the first WHO Expert Committee on the Use of Drugs produced the first edition of WHO Model List of Essential Medicines in 1977 [1], it also recommended the “compilation of a separate list of drugs appropriate for use in primary health care”. Thus the second report of the expert committee [10] produced a separate list of 23 medicines taken from the main list as a model list to be adopted at the national level (Table 1). The report fully adopted the ethos of the Declaration of Alma Ata on PHC in terms of explaining the establishment of PHC systems, respecting the local context, the traditional and existing patterns of health care and the use of these medicines by health workers.
Most of the NEMLs today list the medicines that must be available at the PHC level and aim to make these available in recommended dosage forms all the time. It is also important to mention that PHC requires a proper referral system to secondary and tertiary health care. Together these 3 levels constitute the “basic health care system” in a country. The WHO Model List of Essential Medicines covers all these levels.
The creation of the WHO Drug Action Programme [11] was a significant development between 1979 and 1981 and led to the creation of essential drugs programmes in certain low-income countries. These programmes were established within the context of the national health systems and PHC programmes and began with Tanzania. Some other countries in Asia, Africa and Latin America also established such programmes.
Later came the “selective PHC” approach promoted by agencies like the United Nations Children’s Fund (UNICEF) and United States Agency for International Development (USAID). This gave birth to vertical disease programmes. Along with this there was an ideological change that gradually converted health care as a human right and primary responsibility of the State to a commodity that must be bought by individuals, whether through the public or private sectors. The harbinger of this paradigm shift was the World Bank [12]. These policy shifts fractured the comprehensiveness of PHC and negatively affected community involvement in and ownership of these programmes.
Despite these fundamental changes in health care provision, countries have continued to develop and implement NEMLs. In 1988 WHO came out with guidelines for developing national drug policies [13] (NDPs) which were widely embraced by Member States and country after country began to develop their own NDPs. The concept of essential medicines was a basic tenet of these NDPs. Today more than 100 countries have NDPs based upon this concept. Like WHO itself, most countries review their NEMLs, typically every 2 years, in order to add, delete or change medicines.
PHC, as a first level of health care, if not the comprehensive approach promoted by the Declaration of Alma Ata, continues to use the essential medicine concept in the public sector. But in most low and low-tomiddle income countries the situation in the private sector remains disturbing. The first level of health care in the private sector is still provided by private general practitioners and other allied health professionals, not to mention unqualified practitioners. These, as well as suppliers and sellers of medicines in private pharmacies, are generally not well informed about the concept of essential medicines and they freely prescribe all medicines available in the market. Irrational prescription, adverse drug reactions and high household spending on medicines are all testament to this. However, the emergence of health insurance systems, managed by autonomous institutions or by the private sector, has led to the use of limited lists of medicines, which is actually an application of the essential medicines concept but without the name.
Evolution of the concept of essential medicines
The concept of essential medicines has kept pace with the changing times in terms of evolving public health needs and advancement in medical treatment. It remains as relevant today as it was 30 years ago.
What started as a list of 208 medicines in 1977 has been reviewed 15 times since then. Every 2 years a systematic review is conducted by the WHO Expert Committee on Selection of Medicines. Many medicines have been added to the list, some have been removed and others have been replaced with better alternatives. The 15th edition of WHO Model List of Essential Medicines (2007) consists of 340 medicines, an addition of 132 medicines over 30 years. In 2007 when the Expert Committee met to review the 14th edition of the list its criteria were “due regard to disease prevalence, evidence on efficacy and safety, and comparative cost–effectiveness.”
A major difference in criteria used in the selection of essential medicines has taken place over the years especially from 2002 onwards. The 2nd report of the WHO Expert Committee while laying down the criteria of selection mentioned, “The choice of such drugs depends on many factors, such as pattern of prevalent diseases; the treatment facilities; the training and experience of available personnel; the financial resources; the genetic, demographic and environmental factors.” From 2002 onwards, however, affordability changed from a condition to a consequence of selection. Before 2002, expensive medicines were often not included on the Model List because their inclusion was seen as unrealistic. Under the new definition, a cost-effective medicine can be selected even if the price is high, and the fact that it is considered essential then implies that it has to become available and affordable. The first examples of this new approach were the first-line antiretroviral medicines, which were added to the Model List in 2002 when they were still priced at over US$ 1000 per patient per year; in 2007 they can cost less than US$ 100 per patient per year.
Essential medicines are intended to be available within the context of functioning health systems at all times, in adequate amounts, in the appropriate dosage forms, with assured quality, and at a price the individual and the community can afford.
The implementation of the concept of essential medicines is intended to be flexible and adaptable to many different situations; exactly which medicines are regarded as essential remains a national responsibility [14].
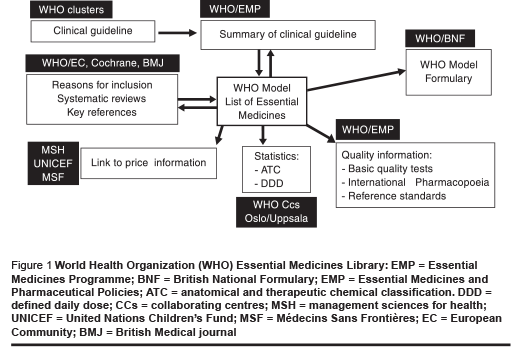
Another important change from 1977 is that now there are a number of associated complementary activities related to essential medicines and based around the Model List of Essential Medicines (Figure 1). The List has been incorporated into the web-based WHO Essential Medicines Library [15], which provides direct links to relevant WHO clinical guidelines, supporting evidence, model formulary text, price information, quality standards and nomenclature.
In 2008 a new WHO Model List of Essential Medicines for Children was published for the first time [16].
Situation in the Eastern Mediterranean Region
Out of 22 countries in the Eastern Mediterranean Region of the WHO, 15 have NEMLs: Afghanistan, Djibouti, Egypt, Islamic Republic of Iran, Iraq, Jordan, Morocco, Oman, Pakistan, Palestine, Somalia, Sudan, Syrian Arab Republic, Tunisia and Yemen. Not surprisingly all the abovementioned countries that have NEMLs also have national medicines policies in place which are explicitly based on the essential medicines concept.
The 7 countries that do not have NEMLs (Bahrain, Kuwait, Lebanon, Libyan Arab Jamahiriya, Qatar, Saudi Arabia and United Arab Emirates) include the Gulf States, except Oman, which are high income countries. Somehow the concept of essential medicines has not taken firm root in the Gulf countries. One of the reasons is that they perceive essential medicines as those needed only in poor countries where resources are scarce and difficult treatment choices have to be made. This is indeed a misunderstanding. However, as insurance systems are now being established in the Gulf countries, they will presumably reimburse only those medicines that are included in the list of the insurance agencies. As mentioned before, this is an application of the essential medicines concept and its principles without the terminology. Other countries in the Region that have health insurance systems established by the government include Egypt, Islamic Republic of Iran, Jordan, Morocco and Tunisia.
The private health insurance sector is increasingly establishing itself in middleand high-income countries in the Region. These private companies also usually have a limited list of fully reimbursable medicines.
Challenges in essential medicines
WHO estimates that over 10 million deaths per year could be avoided by scaling up certain health interventions, the majority of which depend on essential medicines. Yet today, almost 2 billion people do not have regular access to essential medicines; in some of the lowest income countries in Africa and Asia, more than half of the population has no regular access. There are also challenges in conceptualizing and measuring “access to medicines”. The current indicators for measurement neither fully capture the situation nor are they easy to measure.
The global situation is also reflected in the EMR; universal coverage for basic health care, including provision of essential medicines in some of its poor countries, remains elusive. In fact, 11 medicine price surveys in the Region using standard methodology [17] have shown that many important essential medicines are unavailable in public sector health facilities, their generic forms are relatively less available in the private sector and the prices of both generic and proprietary medicines in the private sector are still unaffordable to the poor; this is especially the case in low-income countries.
The essential medicines concept remains limited essentially to the public sector. The private sector, except insurance organizations which are few in low and low-tomiddle income countries, has not embraced the concept for the simple reason that it goes against the business interests of suppliers. It is a daunting but necessary challenge to change this situation, particularly in countries where out-of-pocket spending is high. New business models need to be developed which can encourage the private sector supply of essential medicines.
More and more new essential medicines are needed for existing neglected diseases in developing countries (e.g. trypanosomiasis, leishmaniasis, hepatitis); for newly emerging diseases (e.g. Ebola virus, SARS, avian influenza); and for diseases which are increasingly not responding to existing treatments (e.g. tuberculosis, malaria, HIV/ AIDS). The research and development pipeline is not serving these needs, primarily because these problems affect poor people in poor countries who do not represent an attractive market for big pharmaceutical companies. Therefore, even though these companies enjoy unprecedented levels of intellectual property protection, they are not investing in such research. Innovative ways of promoting research and development directed at these problems are needed; for example patent pools, prize funds, research and development treaties.
Despite the existence of good practices guidelines in selection, procurement, storage and distribution, and use of medicines, supply management of medicines is weak and fragmented in low-income countries because of lack of resources, limited expertise, and minimal accountability and political will. Because of these problems the vertical disease programmes have developed their own supply management systems, and this has resulted in inefficiencies and duplication.
Another major problem is that more than half the medicines are prescribed wrongly and half the patients consume medicines irrationally. This can result in a huge wastage of resources and cause unnecessary suffering through prolonged morbidity and drug injuries. There have been fragmented and ad-hoc efforts to promote rational use of medicines but these have not improved the overall situation in countries and they have not been sustainable. Comprehensive, national and sustained effort is needed to counteract irrational use of medicines.
Conclusion
The concept of essential medicines has proved itself sound, fair and necessary. However, there remain many challenges, the most important being to improve equitable access to those who still suffer unnecessarily for want of essential health care and medicines. PHC together with essential medicines continues to be the most relevant approach to organize and deliver reliable, sustainable and credible health care services in the 21st century.
References
- The selection of essential drugs: report of a WHO expert committee [meeting held in Geneva from 17 to 21 October 1977]. Geneva, World Health Organization, 1977 (WHO Technical Report Series, No. 615):20–30.
- Declaration of Alma Ata (http://www.who. int/hpr/NPH/docs/declaration_almaata. pdf, accessed 3 July 2008).
- Commission on Intellectual Property Rights, Innovation and Public Health. Public health, innovation and intellectual property rights: report of the Commission on Intellectual Property Rights, Innovation and Public Health. Geneva, World Health Organization, 2006.
- Hardman JG, Limbird LE, eds. Goodman & Gilman’s the pharmacological basis of therapeutics, 10th ed. New York, McGraw–Hill, 2001.
- WHO Official Records, No. 226, 1975, Annex 13, pp. 96–110.
- World Health Assembly. Resolution WHA28.66. Geneva, World Health Organization, 1975.
- Quick JD et al. Twenty-five years of essential medicines. Bulletin of the World Health Organization, 2002, 80(11):913– 4.
- Helling-Borda M. Memories of the First Expert Committee Meeting and celebrating 25 years later. Essential drugs monitor, 2003, 32:14–5.
- World Health Assembly. Technical Cooperation. WHA resolution 30.43, 19 May 1977.
- The use of essential drugs: second report of the WHO expert committee [meeting held in Geneva from 3 from 7 December 1984]. Geneva, World Health Organization, 1985 (WHO Technical Report Series, No. 722).
- Mamdani M. Early initiatives in Essential Drugs Policy. In: Kanji N et al., eds. Drugs policy in developing countries. London, Zed Books, 1992:2.
- Ferranti D. Paying for health services in developing countries: an overview. Washington DC, World Bank, 1985.
- Guidelines for developing national drug policies. Geneva, World Health Organization, 1988.
- The selection and use of essential medicines: report of the WHO Expert Committee, 2002 (including the 12th model list of essential medicines). Geneva, World Health Organization, 2003 (WHO Technical Report Series, No. 914).
- The WHO Essential Medicines Library [website] (http://www.who.int/emlib/, accessed 3 July 2008).
- The selection and use of essential medicines: report of the WHO Expert Committee, October 2007 (including the model list of essential medicines for children). Geneva, World Health Organization (in press).
- Medicine prices: a new approach to measurement. Geneva, World Health Organization, 2003.








