Case report
F. Firinci,1 N. Duman,1 O. Ates,2 E. A. Ozer,3 A. Kumral,1 A. Erdemir 3 and H. Ozkan 1
1Department of Paediatrics; 2Department of Paediatric Surgery, Dokuz Eylul University School of Medicine, Izmir, Turkey (Correspondence to F. Firinci:
Cette adresse email est protégée contre les robots des spammeurs, vous devez activer Javascript pour la voir.
,
Cette adresse email est protégée contre les robots des spammeurs, vous devez activer Javascript pour la voir.
).
3Department of Paediatrics, Izmir Tepecik Training and Research Hospital, Izmir, Turkey.
Received: 16/01/12; accepted: 06/12/12
EMHJ, 2013, 19(11):960-961
Introduction
Despite the improvements in prevention of acute respiratory disease in preterm infants, the incidence of bronchopulmonary dysplasia (BPD) remains largely unchanged. Acquired lobar emphysema (ALE) is an increasingly recognized complication of advanced BPD. Barotrauma, oxygen toxicity and lung immaturity are presumed to play an important role in the development of ALE in children with BPD and most cases present overinflation [1,2]. We report on an infant with BPD who developed ALE mimicking pneumothorax.
Case report
Following a 30-week of gestation complicated with premature rupture of membranes for 3 days, a 1275 g male infant was delivered by caesarean section. The initial chest radiography showed a grade IV respiratory distress syndrome (RDS), requiring a total of 3 surfactant administrations. The initial situation was complicated by a systemic inflammatory response syndrome. Ventilatory support was performed to treat respiratory acidosis and severe RDS. On postnatal day 28, when the child had been on mechanical ventilation, a right pneumothorax developed. A chest tube was inserted and removed after 3 days. On postnatal day 31, radiographic evidence of BPD was noted (Figure 1). Although vitamin A supplementation and dexamethasone treatment were administered, the infant did not tolerate extubation. On postnatal day 56, severe acute hypoxaemia developed. Chest radiography confirmed the diagnosis of right pneumothorax and a chest tube was inserted. However, the infant deteriorated clinically and repeated radiography revealed lobar emphysema on the right lower lung. The infant underwent right lower lobectomy and a marked clinical improvement after surgery was evident. The patient was extubated on postnatal day 67. The infant was then transferred to another hospital due to his family’s request on postnatal day 125. On discharge, he was clinically stable and receiving only supplemental oxygen.
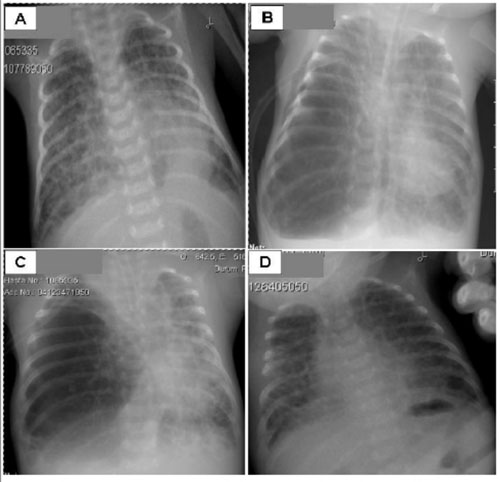
Figure 1 Chest radiographs of a case of acquired lobar emphysema mimicking pneumothorax in a neonate. (A) Postnatal day 21: bilateral diffuse cystic changes in lung parenchyma, consolidation on the left retrocardiac side. (B) Postnatal day56: emphysema on the right lower lobe of the lung, deviation of the heart and mediastinal structures to the left, diffuse cystic parenchymal changes on the right upper lobe and left lobe of the lung. (C) Postnatal day 59: emphysema on the right lower lobe of the lung, deviation of the heart and mediastinal structures to the left. (D) Postnatal day 63: bilateral diffuse cystic parenchymal changes and no emphysema after right lower lobectomy
Discussion
The pulmonary air leak syndromes, including pneumomediastinum, pneumothorax, pulmonary interstitial emphysema and pneumopericardium, comprise a spectrum of disease with the same underlying pathophysiology. They are common in preterm neonates with RDS during treatment with mechanical ventilation. The incidence is about 10% of the ventilated preterm infants treated with surfactants [3]. This is a case report of a preterm newborn developing RDS complicated with BPD and ALE during mechanical ventilation. Initially he was managed with chest tube drainage due to diagnosis of pneumothorax. However repeated radiologic examination revealed the diagnosis of ALE.
ALE is an usual complication of mechanical ventilatory support in neonates with RDS. Although numerous therapeutic approaches to this complication have been described, there is no widely accepted management strategy in current practice. Therapeutic options include positioning of the neonate on the affected hemithorax, selective ventilation of the unaffected lung with conventional ventilation, selective occlusion of the affected mainstem bronchus, surgical resection of the affected lung portion, application of high-frequency ventilation to the trachea and administration of dexamethasone [4–9]. In our case, resection of the large emphysematous bulla was successfully performed without any perioperative complications. Considering the surgical approach the postoperative outcome was encouraging, although the long-term outcome of the child remains unclear. In the literature concerning infants who failed medical management, lobectomy is clearly beneficial [1].
In conclusion, ALE should be kept in mind as a complication in infants with severe BPD on mechanical ventilation. Early diagnosis of the ALE is important for conservative management. A misdiagnosis of pneumothorax should be avoided. Obviously, prevention is better than treatment. Therefore clinical trials in patients with BPD will provide additional therapeutic options for the treatment and prevention of complications of BPD.
References
- Azizkhan RG et al. Acquired lobar emphysema (overinflation): clinical and pathological evaluation of infants requiring lobectomy. Journal of Pediatric Surgery, 1992, 27:1145–1152.
- Miller KE et al. Acquired lobar emphysema in premature infants with bronchopulmonary dysplasia: an iatrogenic disease? Pediatric Radiology, 1981, 138:589–592.
- Ozkan H et al. Synchronized ventilation of very-low-birth-weight infants; report of 6 years’ experience. Journal of Maternal-Fetal and Neonatal Medicine, 2004, 15:261–265.
- Leonidas JC, Hall RT, Rhodes PG. Conservative management of unilateral pulmonary interstitial emphysema under tension. Journal of Pediatrics, 1975, 87:776–778.
- Dickman GI, Short BI, Krauss DR. Selective intubation in the management of unilateral pulmonary interstitial emphysema. American Journal of Diseases of Children, 1977, 131:365.
- Lewis S et al. Pulmonary interstitial emphysema: selective bronchial occlusion with a Swanganz catheter. Archives of Disease in Childhood, 1988, 63:613–615.
- Andreou A et al. One-sided high-frequency oscillatory ventilation in the management of an acquired neonatal lobar emphysema: a case report and review. Journal of Perinatology, 2001, 21:61–64.
- Weintraub Z, Oliven A. Succesful resolution of unilateral pulmonary interstitial emphysema in a premature infant by selective bronchial balloon catheterization. Journal of Pediatric Surgery, 1988, 96:475–477.
- Martin JS et al. Emphyseme lobaire geant acquis chez un premature sous ventilation artificielle. Guerison par la corticotherapie [Acquired giant lobar emphysema in an artificially ventilated premature infant. Cured by corticosteroid therapy]. Annales de Pediatrie, 1993, 40:49–50.



