Z. Kourorian,1 F. Fattahi,1 Z. Pourpak,1 M. Rasoolinejad2 and K. Gholami3
التفاعلات الدوائية الضائرة في أحد أقسام الأمراض المعدية في جمهورية إيران الإسلامية
زهرا كروريان، فاطمة فتاحي، زهرابورباك، مهرناز رسولي نجاد، خير الله غلامي
الخلاصـة: هدفت الدراسة إلى تقييم تكرار وشدة التفاعلات الدوائية الضائرة (وفق تعريف منظمة الصحة العالمية) لدى المرضى الذين أدخلو المستشفى في أحد أقسام الإحالة للأمراض المعدية في طهران. ومن بين 281 مريضاً تم تقييمهم خلال 6 شهور، تم الإبلاغ عن 170 من التفاعلات الدوائية الضائرة المشتبهة لدى 101 مريضاً (%35.9) وقد كان جهاز الهضم هو أكثر الأعضاء تأثراً (%47.5)، وكان أكثر الأدوية المسؤولة عن التفاعلات الدوائية الضائرة شيوعاً مضادات العدوى (%93.1). وقد كانت التفاعلات الدوائية الضائرة مرتفعة بين المرضى الإيجابيين لفيروس الإيدز (%82.9)، ويعود ذلك بشكل رئيسي إلى الأدوية المضادة للسل. ومن المطلوب إيلاء الاهتمام بالوصف الملائم للأدوية مع رصد سريري (إكلينيكي) ومختبري أكثر دقة للمرضى.
ABSTRACT This study aimed to assess the frequency and severity of adverse drug reactions (ADRs) (WHO definition) in hospitalized adult patients in an infectious diseases referral ward in Tehran. Of 281 patients evaluated over 6 months, a total of 170 suspected ADRs were reported among 101 patients (35.9%). The most commonly affected organ system was gastrointestinal (47.5%), and the most common class of drugs responsible was anti-infectives (93.1%). ADRs were high among HIV-positive patients (82.9%), mainly due to anti-tuberculosis drugs. Attention to appropriate prescription of drugs is required with more careful clinical and laboratory monitoring of patients.
Réactions indésirables aux médicaments dans un service iranien de maladies infectieuses pour adultes
RÉSUMÉ Cette étude visait à évaluer la fréquence et la gravité des réactions indésirables aux médicaments (définition de l’OMS) chez des sujets adultes hospitalisés dans un service de maladies infectieuses à Téhéran. Sur 281 patients suivis pendant 6 mois, 170 cas suspects de réactions indésirables aux médicaments (RIM) ont été relevés chez 101 patients (35,9 %). Les organes les plus touchés étaient ceux de l’appareil digestif (47,5 %) et la catégorie de médicaments la plus souvent responsable des RIM était celle des anti-infectieux (93,1 %). Le taux de RIM était élevé chez les personnes séropositives (82,9 %), principalement en raison de la prise d’antituberculeux. Il est nécessaire de veiller à l’adéquation des prescriptions de médicaments et d’effectuer un suivi plus vigilant des patients dans le cadre clinique et en laboratoire.
1Immunology, Asthma and Allergy Research Institute, Children’s Medical Centre; 2Iranian Research Centre for HIV/AIDS, Imam Khomeini Hospital; 3Department of Clinical Pharmacy, College of Pharmacy, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to Z. Pourpak:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
Received: 04/03/07; accepted: 11/07/07
EMHJ, 2009, 15(6):1351-1357
Introduction
Adverse drug reactions (ADRs) are a significant health concern as they exacerbate patients’ morbidity and mortality [1]. The World Health Organization (WHO) definition of an ADR, which has been in use for about 30 years, is “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function” [2]. Thus this definition excludes adverse events caused by errors in drug administration or noncompliance (taking more or less of a drug than the prescribed amount). The percentage of patients experiencing an ADR during hospitalization has been reported to range from 1.5% to 35% [3]. The reported frequency of hospital admissions attributed to ADRs varies from 0.1% to 16.8% [4]. The class of drugs causing the highest number of ADRs is anti-infectives [5].
Although many ADRs are mild and disappear when the drug is stopped or the dose reduced, others may be serious, long-lasting or even life-threatening [6]. An ADR is considered mild when the patient’s normal life is not affected, moderate when it requires inpatient hospitalization or absence from work/school and serious when the ADR is life-threatening or results in persistent or significant disability [7].
Several risk factors have been identified, including previous history of ADR, duration of hospital stay, age, sex, drug exposure, immunodeficiency, chronic disease and liver or renal disease [1,8]. Among
immunodeficiency diseases, HIV-positive individuals, particularly those with advanced immunosuppression, are known to be at increased risk of ADRs [9]. Co-infection with HIV and tuberculosis (TB) is an increasing problem in ADRs [10], with most reactions occurring in the first 2 months of treatment [11].
In order to understand more about the frequency, seriousness and characteristics of ADRs, this study was conducted in a teaching hospital, the main referral centre of infectious diseases and HIV in the Islamic Republic of Iran.
Methods
In this cross-sectional study, 281 patients (189 males and 92 females) aged 18–78 years were assessed over a 6-month period between October 2004 and March 2005. The study was performed in the infectious diseases ward of Imam Khomeini Hospital, a public teaching hospital affiliated to Tehran University of Medical Sciences in Tehran. All patients admitted to this ward were included in the study except repeat admissions and patients with a planned hospitalization of less than 24 hours. Adverse effects caused by errors and/or overdose, drug abuse or therapeutic failures were excluded.
The study protocol was approved by the ethics board of Tehran University of Medical Sciences. Patients were briefed in detail about the study, and informed consent was obtained from all of them.
Two questionnaires were used. The first was filled in for all admitted patients, covering personal information (name, sex, age), date of admission and discharge, cause of admission, history of previous ADR, name and dose of drugs that patients had received before admission and during the hospital stay. All patients were evaluated daily for the presence of ADRs and were followed up until discharge to ascertain the final diagnosis. If a suspected ADR was reported, the second questionnaire was filled in, covering drug treatment, route and duration of drug use, characteristics and evolution of the adverse reaction, duration of hospital stay and time from the start of drug delivery to the start of the manifestations of ADR. Physicians also reviewed charts, laboratory tests and other clinical documents to complete the questionnaire for each patient with a suspected ADR. We separately analysed the rate of ADR occurring in hospital and the rate as a cause of hospital admission.
The reactions (usually more than 1 per patient) were classified by organ system according to the terminology of the WHO ADR monitoring register [12]. A reaction profile was made by calculating the number of reports of each system/organ as a percentage of all the reports. The suspected drugs were classified according to the WHO drugs list [13].
The causality relationship between the ADR and the drug therapy was assessed for each case using the WHO probability scale [14] as: certain (consistent temporal association, including clinical course following withdrawal of drug and, where appropriate, rechallenge); probable (consistent temporal association but not confirmed by rechallenge); possible (likely association but could be explained by another disease or drug); unlikely (temporal relationship to drug administration not consistent with causality); conditional (lack of data necessary for proper assessment but more data being examined); or unassessable (lack of data necessary for proper assessment).
Severity was classified into 4 categories: fatal; severe (directly life-threatening and/or more than 1 month in duration, associated with organ system dysfunction, reduced life expectancy); moderate (some but not all of the mild criteria and none of the severe criteria); or mild (uncomplicated primary disease, no treatment required and drug discontinuation not necessary) [14].
The type of ADR reported was documented using the classification of Rawlins and Thompson: type A (augmentation of the pharmacological actions of a drug) or type B (idiosyncratic, cannot be predicted from the known pharmacology of the drug) [15].
All data from questionnaires were coded for subsequent analysis. All analyses were performed using SPSS for Windows, version 11.0. Continuous data were compared by 1-way analysis of variance and categorical data by the chi-squared test and 2-tailed tests. All measurements are expressed as mean and standard deviation (SD); t-test and paired t-test were used as appropriate.
A P-value < 0.05 was considered to indicate a significant difference.
Results
Of 305 patients admitted to the adult infectious ward during the 6-month period of the study, 24 were excluded because of incomplete information or the presence of study exclusion criteria. Therefore a total of 281 patients (92.1%) were included in the study. The mean age of the patients was 37.9 (SD 16.1) years in males and 44.9 (SD 20.2) years in females (Table 1); 238 patients were aged < 60 years
During the study period, ≥ 1 suspected ADRs were experienced by 101 (35.9%) patients: 71 males (70.3%) and 30 females (29.7%) (Table 1). There was no significant difference in the age distribution of patients with or without ADR. The ADR was the cause of admission to hospital in 8 patients (7.9%) and the ADR occurred in the hospital in 93 patients (92.1%). Suspected ADRs were mild in 40 patients, moderate in 50 and severe in 11.
The total number of suspected ADR swas 170 (i.e. 1.68 per patient): 50 (49.5%) patients developed 1 ADR; 31 (30.7%) patients 2 ADRs and 20 (19.8%) patients ≥ 3 ADRs. The ADRs were classified as 25 certain reactions, 109 possible reactions and 36 probable reactions. Most ADRs (76.2%) were classified as type A.
The drug class most commonly implicated in ADRs was anti-infectives (93.1%); the most common were 3rd-generation antibiotics (ceftriaxon, cefazolin), followed by amoxicillin, sulfamethoxazole and anti-TB medicines (including rifampin and isoniazid). There were 41 patients (14.6%, all males) with HIV infection; 34 (82.9%) had an ADR, mainly caused by the anti-TB medication.
Almost all patients (96.0%) who developed an ADR were receiving > 2 anti-infective drugs. Mean number of drugs taken was 4.3 (SD 2.2) at the time of experiencing an ADR. A significant relationship was found between the number of drugs administered and the frequency of ADRs (P = 0.002).
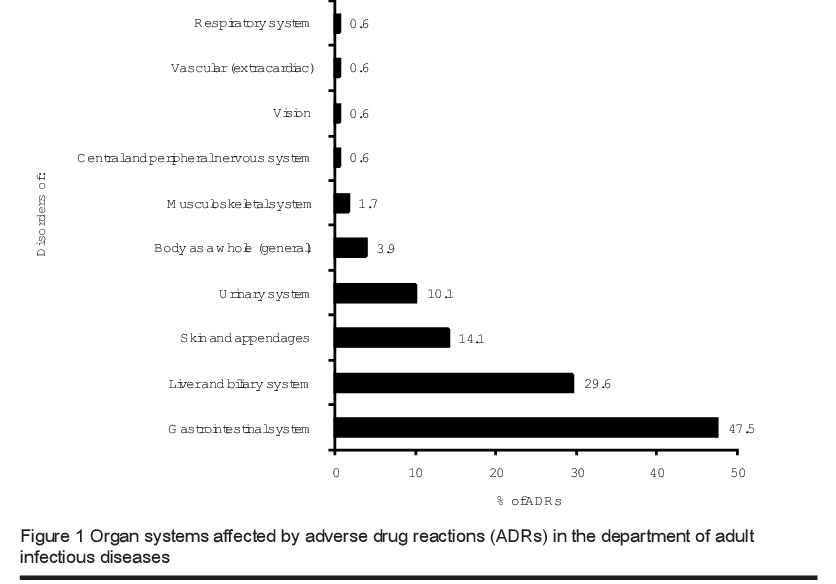
The organ systems most commonly involved in ADRs were gastrointestinal (47.5%), followed by liver (29.6%) and skin (14.1%) (Figure 1). The most common clinical manifestations of ADRs during the study period were diarrhoea and distention, nausea with or without vomiting (48 patients) and increased alanine transaminase and aspartate aminotransferase levels (30 patients) that induced mild symptoms such as malaise, nausea and anorexia. No patients had jaundice or elevation of bilirubin level. Skin manifestations (mainly in the form of rashes) were 34 detected in 15 cases.
The mean length of stay in hospital was 11.8 days. Duration of hospitalization was significantly longer in patients who experienced an ADR (14.6 days versus 10.2 days)
(P < 0.001). There was no significant association between duration of hospitalization and age. All patients recovered without severe sequelae.
Discussion
ADRs have been the subject of many studies, taking into account different aspects of their complications such as the methodology used to detect ADRs, patients’ age, hospitalization, costs and prolonging hospital stay. Although the findings of these studies usually represent similar patterns for recognizing and reducing ADRs and therefore safe and rational use of medicinal products, there are differences between the rates of ADRs reported. These differences may depend on the definition used for an ADR, the study population and medications used for treatment. We used the more conservative WHO definition of an ADR that avoids overestimating the ADR rate [1,2].
The frequency of ADR in our study was 35.9%, showing that ADRs were in the highest frequency category according to WHO guidelines, which recommend estimating the frequency from “very rare” (< 0.01%) to very common (> 10%) [16].
Most ADRs were classified as type A, i.e. predictable from the pharmacology of the drug. It has been shown that careful drug monitoring in hospitals leads to a reduction in many of these, suggesting that some type A ADRs may be due to inadequate monitoring of therapies and doses.
As in other ADR studies [17,18], our study focused on ADRs induced by anti-infective drugs in hospitalized patients. Over the 6-month evaluation period, the rate of ADRs in our infectious diseases department was 35.9%. This is higher than the rate found in similar studies using the WHO definition by Ramesh et al. (7.2%) [6] and Gholami et al. (8.2%) [19]. One explanation for our much higher rate is the presence of patients who had HIV and TB.
We found no significant association between age or sex and the occurrence of ADRs. Several community- and hospital-based studies have found female sex to be a risk factor for ADRs or admissions to hospital caused by ADRs [8]. However, the data are conflicting. For example, Mitchell et al. showed that 2% of admissions at 2 teaching hospitals and 3 community hospitals were prompted by ADRs and the number of admissions and also the number of ADRs was greater in males [20].
In our study, the age distribution of patients with an ADR was similar to patients without an ADR. Many studies from around the world showed a correlation between increasing age and the ADR rate [21].
Our data showed a significant association between the number of medications received by patients and risk of an ADR. Other studies also clearly show that the risk of ADRs (including interactions) is related to the number of medicines [14,22].
There was a high frequency of ADR (82.9%) among HIV-positive patients. This is not surprising because they are known to be at increased risk [9].
High consumption of antibiotics and multiple drug exposure is an important risk factor for ADRs [1]. In our study, as in others, more than half the patients received anti-infective agents, which are one of the leading classes of drugs that induce ADRs and account for one-third to one-half of the pharmacy budgets of most inpatient facilities [23]. Nonetheless, there is a consensus that excessive and inappropriate use of anti-infective agents is a global problem that not only adds a substantial economic burden to the health care system but also contributes to the selective pressures favouring the development of resistance [24].
Some limitations of the study design may have influenced the results. First, our study was performed in an infectious diseases ward, where there was a high consumption of anti-infective agents, and bias exists because of this specific ward type. Second, because data were collected over the autumn and winter but not for a 1-year period it is possible that seasonal variations may have influenced the frequency of ADRs reported.
Nevertheless this study may increase awareness of ADRs among physicians, pharmacists and other medical staff, and encourage them to be move vigilant in monitoring patients who take anti-infective agents as part of their drug therapy and to remind them to consider these reactions in the differential diagnosis. The simplest way to prevent most ADR is attention to appropriate prescription of drugs with more careful clinical and laboratory monitoring of patients. Physicians should be especially alert to the synergistic effects in patients suffering co-infection with HIV and TB.
Acknowledgements
We offer our special thanks to Drs G. Shalviri, A. Kazemnejad, Z. Ahmadinejad and M. Moin, our colleagues in the ADR research group, and the residents and nurses of the infectious diseases ward in Imam Khomeini Hospital for their collaboration in this study.
References
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Journal of the American Medical Association, 1998, 279(15):1200–5.
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet, 2000, 356(9237):1255–9.
- Dormann H et al. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug safety, 2000, 22(2):161–8.
- Muehlberger N, Schneeweiss S, Hasford J. Adverse drug reaction monitoring—cost and benefit considerations. Part I: frequency of adverse drug reactions causing hospital admissions. Pharmacoepidemiology and drug safety, 1997, 6(Suppl. 3):S71–7.
- Gholami K et al. Anti-infectives-induced adverse drug reactions in hospitalized patients. Pharmacoepidemiology and drug safety, 2005, 14(7):501–6.
- Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital––their severity and cost involved. Pharmacoepidemiology and drug safety, 2003, 12(8):687–92.
- Ayani I et al. A cost-analysis of suspected adverse drug reactions in a hospital emergency ward. Pharmacoepidemiology and drug safety, 1999, 8(7):529–34.
- Montastruc JL et al. Gender differences in adverse drug reactions: analysis of spontaneous reports to a Regional Pharmacovigilance Centre in France. Fundamental & clinical pharmacology, 2002, 16(5):343–6.
- Kingston M, Childs K, Carlin E. Adverse reaction to antimycobacterials administered as a combination tablet with no reaction to the same drugs in isolation. Sexually transmitted infections, 2001, 77(5):392–3.
- Harb GE et al. Pharmacoepidemiology of adverse drug reactions in hospitalized patients with human immunodeficiency virus disease. Journal of acquired immune deficiency syndromes, 1993, 6(8):919–26.
- Narain JP, Lo YR. Epidemiology of HIV-TB in Asia. Indian journal of medical research, 2004, 120(4):277–89.
- International monitoring of adverse reactions to drugs: adverse reaction terminology. Uppsala, Sweden, World Health Organization Collaborating Centre for International Drug Monitoring, 2002.
- Essential medicines. WHO model list (revised April 2003): explanatory notes, 13th ed. Geneva, World Health Organization, 2003.
- Fattahi F et al. Adverse drug reactions in hospitalized children in a department of infectious diseases. Journal of clinical pharmacology, 2005, 45(11):1313–8.
- Rawlins MD, Thompson JW. Mechanisms of adverse drug reactions. In: Davies DM et al., eds. Textbook of adverse drug reactions. Oxford, Oxford University Press, 1991:18–45.
- Frequency of adverse drug reactions. In: Guidelines for preparing core clinical safety information on drugs. Report of CIOMS Working Group III. Geneva, World Health Organization, 1995.
- May JR. Adverse drug reactions and interactions. In: DiPiro JT et al., eds. Pharmacotherapy, a pathophysiologic approach. Stanford, Connecticut, Appleton and Lange, 1997:101–6.
- Hallas J. Drug related hospital admissions in subspecialities of internal medicine. Danish medical bulletin, 1996, 43(2):141–55.
- Gholami KH, Shalviri G. Factors associated with preventability, predictability and severity of adverse drug reactions. Annals of pharmacotherapy, 1999, 33(2):236–40.
- Mitchell AA et al. Adverse drug reactions in children leading to hospital admission. Pediatrics, 1988, 82(1):24–9.
- Routledge PA, O’Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. British journal of clinical pharmacology, 2004, 57(2):121–6.
- Lassila HC et al. Use of prescription medications in an elderly rural population: the MoVIES Project. Annals of pharmacotherapy, 1996, 30(6):589–95.
- Classen DC et al. Computerized surveillance of adverse drug events in hospital patients. Journal of the American Medical Association, 1991, 266(20):2847–51.
- Evans RS et al. A computer-assisted management program for antibiotics and other antiinfective agents. New England journal of medicine, 1998, 338(4):232–8.



