M.A. Moustafa,1 H.S. Thabet,1 G.A. Saad,1 M. El-Setouhy,2 M. Mehrez3 and D.M. Hamdy1
ترصد داء الخيطيات اللمفاوي بعد خمس سنوات من إيقاف إعطاء الأدوية الجموعي في محافظة المتوفية، مصر
منال عبد العزيز مصطفى، هالة صبحي ثابت، غادة عبد الرحمن سعد، ماجد السطوحي، محمد محرز مصطفى، دينا ممدوح حمدي
الخلاصة: توصي منظمة الصحة العالمية بإجراء مسوحات إضافية بعد مرور 5 سنوات عل الأقل عل إيقاف الإعطاء الجموعي للأدوية قبل تأكيد التخلص من داء الخيطيات اللمفاوي. وتهدف الدراسة الحالية إلى التعرف على الوضع الذي آل إليه داء الخيطيات اللمفاوي بعد مرور 5 سنوات على إيقاف إعطاء الأدوية الجموعي في 3 قرى خافرة في محافظة المنوفية في مصر. واستخدم الباحثون اختبار البطاقة السريعة للاستشراب المناعي ICT، وحقيبة تجارية لكشف الأضداد هي سيب(®CELISA). واتضح للباحثين أن جيع الأطفال في المرحلة الابتدائية والذين تزاوح أعمارهم بين 6-7 متوات وعددهم 1321 طفلأ كانوا سلبيين، وأن هناك 27 طفلاً لديهم إيجابية في الأضداد. كما أن جميع الأسر التي أجري المسح عليها في إحدى القرى التي كانت الأعلى من حيث معدل انتشار الأضداد كانوا سلبيين باختبار البطاقة السريعة الاستشراب المناعي، مما يشير إلى غياب داء الخيطيات اللمفاوي. أما حقيبة سيليسا التجارية فتحتاج إلى المزيد من المعايرة والتطور حتى تصبح مفيدة في العمل الميداني. واستنتج الباحثون أن داء الخيطيات اللمفاوي لم يعد من مشكلات الصحة العامة في هذه القرى وفي القرى الأخرى التي تشابها في الظروف الوبائية.
ABSTRACT The World Health Organization recommends that before lymphatic filariasis elimination in an area can be confirmed, an additional survey should be performed at least 5 years after stopping mass drug administration. The current study aimed to determine the status of lymphatic filariasis 5 years after cessation ofthe mass drug administration in 3 sentinel Egyptian villages in Menoufiya Governorate. The rapid immunochromatographic card test (ICT) and a new commercial antibody detection kit (CELISA®) were used. All 1321 primary-school children aged 6-7 years old were ICT negative but 27 children were antibody positive. All households surveyed in one village with the highest antibody prevalence were ICT negative, indicating an absence of lymphatic filariasis. The CELISA antibody kit needs more standardization and development to be useful under field conditions. We conclude that lymphatic filariasis is no longer a public health problem in these villages and other villages with similar epidemiological conditions.
Surveillance de la filariose lymphatique cinq ans après l'arrêt de l'administration massive de médicaments dans le Gouvernorat de Menoufiya (Egypte)
RÉSUMÉ L'Organisation mondiale de la Santé recommande de mener une enquête supplémentaire au moins cinq ans après l'arrêt de l'administration massive de médicaments avant de confirmer l'élimination de la filariose lymphatique dans une zone donnée. La présente étude visait à déterminer le statut de la filariose lymphatique cinq ans après l'arrêt de l'administration massive de médicaments dans trois villages sentinelles égyptiens du Gouvernorat de Menoufiya. Le test immunochromatographique sur carte (ICT) rapide et un nouveau kit de détection d'anticorps commercial (CELISA®) ont été utilisés. L'ensemble des 1321 écoliers du primaire âgés de 6 à 7 ans avaient des résultats négatifs à l'ICT mais 27 enfants avaient des résultats positifs aux anticorps. Tous les ménages qui ont fait l'objet d'une enquête dans un village où la prévalence des anticorps était la plus élevée ont eu des résultats négatifs à l'ICT, ce qui indique une absence de filariose lymphatique. Le kit de détection d'anticorps CELISA doit faire l'objet d'un développement et d'une normalisation plus poussés pour être utile dans des conditions de terrain. Nous en concluons que la filariose lymphatique ne représente plus un problème de santé publique dans ces villages ainsi que dans d'autres villages ayant des conditions épidémiologiques similaires.
1Department of Medical Parasitology; 2Department of Public Health, Faculty of Medicine, Ain Shams University, Cairo, Egypt (Correspondence to M.A. Moustafa:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
3General Departmentfor Malaria, Filariasis and Leishmaniasis Control, Endemic Diseases Control Sector, Ministry of Health, Cairo, Egypt.
Received: 04/10/13; accepted: 25/11/13
EMHJ, 2014, 20(5): 295-299
Introduction
Lymphatic filariasis is a globally distributed disease recognized by the World Health Organization (WHO) as one of the most disabling diseases. Bancrof-tian filariasis, caused by the parasite Wuchereria bancrofti and transmitted to humans by mosquitoes, accounts for more than 90% of this disease burden [1]. Following the World Health Assembly resolution in 1997 calling for global elimination of lymphatic filariasis in disease-endemic areas by the year 2020 [2,3], the Egyptian Ministry of Health and Population initiated a national programme in 2000 to eliminate lymphatic filariasis [4]. Directly observed mass drug administration (MDA) was conducted in all known filariasis-endemic localities in the country, with annual doses of diethylcarbamazine (6 mg/kg) with albendazole (400 mg) to the whole at-risk population (more than 2. 5 million people) over a 2-week period every year for 5 years [5]. Studies in Egypt have suggested the following provisional targets for treated populations at least 5 years after stopping MDA: < 2% for antigenaemia (which corresponds to a microfilariae prevalence of < 0.5%), < 2% for antibody prevalence in first-year primary schoolchildren and < 0.25% for mosquito infection rates by molecular xenomonitoring [6]. However, given the uncertainty regarding the number of rounds ofMDA that are actually needed to achieve the elimination of lymphatic filariasis [7], surveillance is required as a key component of the elimination programme [8].
Weil and Ramzy discussed the value of different diagnostic tests (serum antigen and antibody assays and detection of parasite DNA in vector mosquitoes) for different phases oflymphatic filariasis elimination programmes [9]. They have also reported the usefulness of filarial antibody tests for identifying endemic areas and following antibody rates in young children over time as a mean of assessing changes in transmission rates following MDA [6,8]. The very slow decline of serum antibodies to the Bm14 gene in treated individuals allows for the use of Bm14 enzyme-linked immunosorbent assay (ELISA) in serial surveys of young children to assess changes in lymphatic filariasis transmission following MDA [9,10]. Therefore, the use of more sensitive antibody tests to detect filarial exposure will be useful to assess transmission at the terminal stages of lymphatic filariasis programmes [9,11]. The CELISA" Bm14 antibody assay has been commercially available as a diagnostic tool since 2006, yet application to large population sizes in field studies has not been thoroughly assessed [9]. In a multicentre laboratory-based study Weil et al. concluded that this kit appeared to be an excellent test for the specific detection ofantibody responses to filarial parasites. They recommended the use of this antibody tool as a practical monitoring option for late stages of filariasis elimination programmes and for post-MDA surveillance [12].
WHO has recommended that before lymphatic filariasis elimination in an area can be confirmed, at least one additional survey should be performed at least 5 years after stopping MDA [13]. Based on these recommendations, the aim of our study was to determine the status oflymphatic filariasis 5 years after cessation ofMDA in 3 sentinel Egyptian villages in Menoufiya Governorate. We conducted a transmission assessment survey in which primary schoolchildren (6-7 years of age) were tested using an immunochromatographic card test (ICT) as recommended by WHO [14], in addition to use of the antibody detection tool, the Bm14 CELISA, as a new surveillance tool.
Methods
Study setting and sample
This study was conducted in 3 sentinel villages (village A: Abo Sneita; village B: Garawan; village C: Kafr El Bagour) located in El Bagour district of Menou-fiya Governorate. These villages were known to have a high prevalence of microfilaraemia in Egypt just before initiation of the Global Programme to Eliminate Lymphatic Filariasis.
A transmission assessment survey was conducted whereby the study population was all the available primary-school children (6-7 years of age) in all schools in the 3 villages (n = 1321). This choice was based on the fact that these children were born immediately after lymphatic filariasis transmission had likely been interrupted and cessation of the MDA after 5 rounds. Thus this age group was a sensitive indicator of the presence of ongoing transmission of lymphatic filariasis. The study started in November 2010 and ended in December 2010.
In February 2012, a household survey was done. It included members of families ofprimary-school children with CELISA positive results in Abo-Sneita village (aged 16-60 years, n = 40), and a limited number of random blood samples were taken from different sectors of the village (age 16-60 years, n = 35), making a total of 75 cases.
Data collection
Two tests for the diagnosis of filariasis were performed on a total of 1321 primary-school children in grade 1 (n = 632) and grade 2 (n = 689): the ICT and the ELISA test for IgG4 antibody to the Bm14 recombinant filarial antigen. Subjects in the household survey were tested by ICT card test only.
For the detection of antigenaemia by ICT (Binax ICT filariasis cards, 620-000) a 300 finger-prick blood sample was collected from each study participant. The ICT test was performed on 100 of the blood for the presence or absence of circulating filarial antigen whereby the blood was drawn onto the card and the results were read visually (negative/positive) after 10 minutes. The remaining 200 finger-prick blood was used to prepare plasma samples for the performance of the Bm14 ELISA using the filariasis CELISA" kit (Cellabs Pty Ltd). ELISA was performed as recommended by instructions from the developers of the assay. Aliquots of plasma diluted 1:100 were tested in parallel for IgG4 antibody to Bm14 and a positive control. It was more accurate to take the readings of all reactant wells without blanking the plate reader. The absorbance of each well was read at optical density (OD) 450 nm. The cut-off value was calculated as the mean plus 3 standard deviations (+ 3 SD) of known negative samples. The readings of test samples were calculated based on the differential absorbance of unknown versus known negative samples. In this study, the cut-off value was 0.4 OD units. Thereafter, any sample showing OD < 0.4 was considered negative, while any sample showing OD > 0.4 was considered positive.
The study was approved by the ethical committee of scientific research at Ain Shams University Faculty of Medicine, and written consent from children's guardians or school headmasters was obtained, according to National Institute of Health guidelines.
Data analysis
The data were analysed using SPSS, version 17, and the chi-squared test was used to assess differences between categories. P-values < 0.05 were considered significant and F = 2 indicating analysis of variance.
Results
All 1321 schoolchildren from the 3 villages and 75 adults were negative by ICT (0%). Among the 1321 students, 29 (2.2%) were positive in the CELISA (Figure 1).
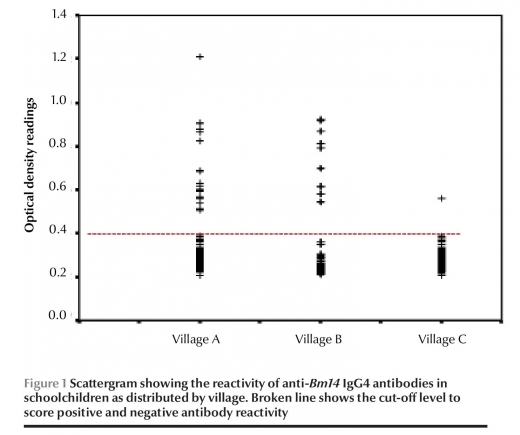
The prevalence of anti-Bmi4 antibodies varied considerably between villages (Table 1). This variation was statistically significant (x2 = 57.6, P < 0.001). There was a higher rate of positive ELISA among students in village A (9.0%) compared with village B (1.9%) and village C (0.2%). Also, there was a higher mean OD among students in village A (0.314) compared with that villages B and C (0.251 and 0.253 respectively) (Table 2).
Discussion
The aim of this study was to determine the status of lymphatic filariasis 5 years after cessation of MDA in 3 sentinel Egyptian villages in Menoufiya Gov-ernorate. In conducting the study, we tested primary-school children using the ICT test as recommended by WHO and a new antibody detection tool, the Bm14 CELISA. Regarding the ICT test, no positive cases (either schoolchildren or household members) were detected, and 100% of the study subjects showed negative results. The ICT card test is a technique recommended by WHO [13] for detection of circulating W. bancrofti antigens to assess the progress towards elimination endpoints in children born when disease transmission has been significantly reduced or interrupted following MDA. According to a study done in Giza (an area of high pre-MDA antigenaemia) and Qalyubia (an area of low pre-MDA antigenaemia) [6], antigen prevalence rates fell by 75% and 77% respectively about 7-10 months after the 5th round of MDA. The present study was carried out 5 years after stopping MDA, which allowed more time for filarial antigenae-mia to vanish.
The finding that all tested schoolchildren aged 6-7 years were ICT negative clearly indicates interruption of transmission and possible elimination of lymphatic filariasis. The goal of the Global Programme is to eliminate lymphatic filariasis as a public health problem by reducing the transmission of filariasis to a very low level so that the transmission will cease to occur in time provided there is no reintroduction of transmission. However, it is possible that the sensitivity of the ICT card test in low-transmission settings might not be sufficient to detect low-level infections [15]. Also previous studies have shown that pre-MDA antibody prevalence rates are much higher than antigen prevalence rates in low-prevalence Egyptian villages [16].
Regarding the Bm14 antibody assay, 2.2% of the study subjects (1321 schoolchildren) showed positive results, and the highest percentage of positive cases (9.0%) was in Abo Sneita village. Therefore the antibody prevalence rate has risen from 0.28% just after the end of the 5th round of MDA to 9.0% 5 years after the elimination programme in Abo Sneita [17]. This contradicting finding should be analysed carefully in order to determine accurately the infection status of this village. Almost all filariasis CELISA-positive samples showed low reactivity (OD values > 0.4-< 1.0). These may be due either to false positives or flagging early exposure to infection.
Several other publications have evaluated the IgG4 antibody detection to the rBm14 antigen as a monitoring tool post-MDA; however, most of them have obtained the rBm14 antigen directly from Dr Gary Weil's laboratory in Washington DC before Cellabs Pty Ltd of Australia developed the new kit (CELISA) in 2006 [6,18,19]. A study conducted by Ramzy just after the 5th round of MDA in 2 Egyptian villages reported that the antibody prevalence rates can take several years to fall to low levels after MDA [6]. Their data suggest that Bm14 antibody may be a sensitive test to monitor continuing residual transmission during and after MDA.
To the best of our knowledge, the commercial filariasis CELISA has not been applied in field studies; therefore, no comparable data are available. A household survey (the 2nd section of our study) using ICT testing in Abo Sneita village (village A) was essential in order to confirm or deny resurgence of lymphatic filariasis and also to solve the puzzle of the CELISA kit results. We suggest that this is a much easier and more practical approach for investigating an area for lymphatic filariasis resurgence after MDA rounds rather than screening of all houses, and also is an appropriate method of field evaluation of the tools used in post-MDA monitoring. The target population in household survey were older residents in Abo Sneita village (mostly households of CELISA antibody-positive children), based on the fact that they had participated in the lymphatic filariasis elimination programme over 5 years, as confirmed by them. Therefore, they are a good indicator ofresidual infection in the village. However, no positive card test results were observed in any of the study subjects, which suggests there were no cases with active infection and refutes the hypothesis that resurgence of lymphatic filariasis has occurred in this area.
On the other hand the new commercial ELISA kit has been tested in a multicentre laboratory evaluation [12], and it was reported that the Bm14 CELISA appears to be an excellent test for the specific detection of antibody responses to filarial parasites and its high sensitivity makes antibody testing especially useful as an epidemiologi-cal tool for assessing levels of infection and/or exposure to filarial parasites in populations. In this study, the raised suspicion that there may be a focus of resurgence in Abo Sneita village, based on the results of the new commercial ELISA, was contradicted by the results of the household survey showing no positive cases detected by ICT. More research is needed to determine the relative value ofantibody and molecular xenomonitoring testing as monitoring tools. Each has advantages, and the 2 approaches are complementary. Antibody monitoring provides information on the cumulative lifetime exposure to filarial infection, while molecular xenomonitoring provides information on the point prevalence of filarial parasites in mosquitoes in the area of interest [20].
In conclusion, our results provide evidence that 5 rounds of MDA with diethylcarbamazine and albendazole has improved the various measures of filariasis endemicity and transmission in these 3 sentinel Egyptian villages, and demonstrates the success of the national programme to eliminate lymphatic filariasis in Egypt. Moreover, the transmission assessment survey has provided satisfactory results concerning the elimination status of the disease in these areas. The new Bm14 antibody detection kit needs further field evaluation using control groups including older children (5th grade primary students) and further development by the manufacturers in order to be a standard and valid monitoring tool under field conditions as for the ICT. In addition, more field studies in different epidemiological settings are required for validation of the filariasis CELISA as a surveillance tool. The use of molecular xenomonitoring in conjunction with ICT would be beneficial in assessment of lymphatic filariasis transmission in the villages under study.
Acknowledgements
The sponsor of the study reviewed the study protocol to ensure compliance with good clinical practice standards. Otherwise, the sponsor had no role in the study design, data collection, data analysis and data interpretation. Precious assistance was provided by the Egyptian Ministry of Health and Population.
Funding: This work was sponsored by the WHO Regional Office for the Eastern Mediterranean, in collaboration with the Special Programme for Research and Training in Tropical Diseases (TDR), Joint EMRO/TDR Small Grants Scheme for implementation research in communicable diseases (grant no. ID SGS 10/43).
Competing interests: None declared
References
- Molyneux D. Lymphatic filariasis: exemplifying the research, policy and practice continuum in a global programme. Paper presented at Forum 77. Equitable access@ research challenges for health in developing countries, Beijing, China, 29 October-2 November2007. Geneva, Global Forum for Health Research, 2007 (http://www.epidemiologia.anm.edu.ar/ pdf/publica-ciones_cie/2007/ART3.pdf, accessed 31 January 2014).
- Ottesen EA et al. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Neglected Tropical Diseases, 2008, 2:e317.
- Report of the Working Group on Guidelines for Stopping MDA and Post-MDA Surveillance for the Elimination of Lymphatic Filariasis. 7-8 August 2008. Geneva, World Health Organization, 2008.
- Global Programme to Eliminate Lymphatic Filariasis. Annual report, 2002. Geneva, World Health Organization, 2003 (http://whqlibdoc.who.int/hq/2003/WHO_CDS_CPECEE_2003.38.pdf, accessed 31 January 2014).
- The global elimination of lymphatic filariasis—the story of Egypt. Geneva, World Health Organization, 2003 (WHO/CDS/ CPE/2003.1) (http://www.who.int/iris/handle/10665/68372, accessed 31 January 2014).
- Ramzy RMR et al. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet, 2006, 367:992-999.
- Kyelem D et al. Determinants of success in national programs to eliminate lymphatic filariasis: a perspective identifying essential elements and research needs. American journal of Tropical Medicine and Hygiene, 2008, 79:480-484.
- Weil GJ et al. The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on bancroftian filariasis in Papua New Guinea. PLoS Neglected Tropical Diseases. 2008, 2(12):e344.
- Weil GJ, Ramzy RM. Diagnostic tools for filariasis elimination programs. Trends in Parasitology, 2007, 23:78-82.
- Helmy H et al. Bancroftian filariasis: effect of repeated treatment with diethylcarbamazine and albendazole on microfila-raemia, antigenaemia and antifilarial antibodies. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2006, 100:656-662.
- Lammie P et al. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis—a multicenter trial. Filaria journal, 2004, 3(9). doi: 10.1186/14752883-3-9.
- Weil GJ et al. A multicenter evaluation of a new antibody test kit for lymphatic filariasis employing recombinant Brugia ma-layiantigen Bm-14. Acta Tropica, 2010, 120(Suppl. 1):S19-S22.
- Monitoring and epidemiological assessment of the programme to eliminate lymphatic filariasis at implementation unit level. Geneva, World Health Organization, 2005 (WHO/CDS/CPE/ CEE) (http://whqlibdoc.who.int/hq/2005/who_cds_cpe_ cee_2005.50.pdf, accessed 31 January 2014).
- Monitoring and epidemiological assessment of mass drug administration in the global programme to eliminate lymphaticfila-riasis: a manual for national elimination programmes. Geneva, World Health Organization, 2005:23. (WHO/HTM/NTD/PCT/2011.4).
- Huppatz C et al. Lessons from the Pacific programme to eliminate lymphatic filariasis: a case study of 5 countries. BMC Infectious Diseases, 2008, 9:92. doi: 10.1186/1471-2334-9-92.
- Ramzy RM et al. Evaluation of a recombinant antigen-based antibody assay for diagnosis of bancroftian filariasis in Egypt. Annals of Tropical Medicine and Parasitology, 1995, 89:443-446.
- Ramzy RM. Has Egypt eliminated lymphatic filariasis? Paperpre-sented at the conference of the Egyptian Parasitologists United Society on 6April2070. Cairo, Egypt, Ain Shams University, 2010.
- Mladonicky JM et al. Assessing transmission of lymphatic filariasis using parasitologic, serologic, and entomologic tools after mass drug administration in American Samoa. American Journal ofTropical Medicine and Hygiene, 20 09, 80:769-773.
- Tisch DJ et al. Mass drug administration trial to eliminate lymphatic filariasis in Papua New Guinea: changes in microfilaremia, filarial antigen, and Bm14 antibody after cessation. American journal of Tropical Medicine and Hygiene, 2008, 78:289-293.
- Weil GJ. Diagnostic tools for filariasis elimination programmes. In: Report of the Scientific Working Group meeting on Lymphatic Filariasis, Geneva, 70-72May2005. Geneva, Switzerland, World Health Organization, 2005 (TDR/SWG/05) (http:// www.who.int/tdr/publications/publications/swg_lymph_fil. htm accessed 31 January 2014).



