Z. Gezelbash,1 H. Vatandoost,1,2 M.R. Abai,1 A. Raeisi,1 Y. Rassi,1 A.A. Hanafi-Bojd,1 H. Jabbari 2 and F. Nikpoor 1
التقييم المختبري والميداني لمستحضرين من العُصَيَّة الثورنجية M-H-14 ضد يرقات البعوض في جمهورية إيران الإسلامية، 2012
زهرا قزلباش، حسن وطن دوست، محمد رضا عبائي، أحمد رئيسي، ياور راثي، أحمد علي حنفي بجد، حسين جباري، فاطمة نيكبور
الخلاصة: في ضوء تضاؤل الكفاءة للمنتج التجاري المحلي المستحضر من العُصية الثورنجية صمم الباحثون دراسة لتقييم كفاءته في المختبر ضمن حوض مائي زجاجي معياري، وفي ظرف ميدانية وشبه ميدانية لكل من الجرعات المستهدفة والجرعات الأعلى ضد المراحل غير الناضجة من أنواع الأنوفيلات وأنواع الباعضات. وفي الظروف المختبرية، كانت قيم الجرعة القاتلة للنصف من مستحضرات المسحوق المرطَّب جزءاً بالمليون ومن الحبيبات جزءاً بالمليون، ضد الذراري المستجيبة من الأنوفيلة الأسطفانية. وبعد تطبيق المسحوق المرطّب والحبيبات على حوض مائي بمقدار 65.1 مغ/حوض وجد الباحثون أن معدل بقاء اليرقات على قيد الحياة بعد تعرضهن لمستحضر الحبيبات كان 65.6 % في اليوم 6، و54.2 % في اليوم 8. أما في الأحواض الاصطناعية فإن كثافة اليرقات انخفضت إلى 38.9 % بجرعة 2 غرام/متر مربع، وإلى 39.3 بجرعة 4 ميلي غرام، وإلى 65 % بجرعة 8 غرام/متر مربع. وفي حقول زراعة الأرز فقد أدت جرعة مقدارها 2 كيلو غرام/هيكتار إلى انخفاض كثافة اليرقات غير الناضجة إلى 33.1 % و28.6 % بعد مرور 7 أيام على المعالجة. وهناك حاجة لمزيد من الدراسات حول أسباب انخفاض كفاءة مبيدات اليرقات.
ABSTRACT Due to low efficacy in the field of a local commercial product of Bacillus thuringiensis M-H-14 (Bioflash®), a study was designed to assess its efficacy in laboratory, glass standard aquarium, semi-field and field conditions at both target and higher dosages against immature stages of Anopheles spp. and Culex spp. In laboratory conditions, the LC50 values of wettable powder and granule formulations were 227 and 1031 ppm respectively against a susceptible strain of An. stephensi. Following application of wettable powder and granules at 56.1 mg/aquarium, the survival rates of the exposed larvae to the granule formulation were 65.6% and 54.2% on days 6 and 8 respectively. In the artificial ponds, the larval density was reduced to 38.9%, 39.3% and 65.1% at dosages of 2, 4 and 8 g/m2 respectively. In rice fields, at a dosage of 2 kg/ha, the density of immature larvae were reduced to 33.1% and 28.6% 7-days post-treatment. Further investigations are needed for the reasons for the low efficacy of this larvicide.
Évaluation en laboratoire et sur le terrain de deux formulations de Bacillus thuringiensis M-H-14 contre les larves de moustiques en République islamique d'Iran, 2012
RÉSUMÉ En raison de la faible efficacité sur le terrain d'un produit commercial local de Bacillus thuringiensis M-H-14 (Bioflash®), une étude a été élaborée pour évaluer son efficacité en laboratoire, en aquarium de verre standard ainsi que dans des conditions de terrain et de semi-terrain à des doses cibles mais aussi à des doses supérieures contre des Anopheles spp. et des Culex spp. à des stades immatures. Aux conditions de laboratoire, les valeurs de la CL50 pour la poudre mouillable et les formulations en granulés étaient de 227 et 1031 ppm respectivement sur une souche sensible d'An. stephensi. Après l'application de poudre mouillable et de granulés à 56,1 mg/aquarium, les taux de survie des larves exposées à la formulation en granulés étaient de 65,6 % et 54,2 % aux jours 6 et 8 respectivement. Dans les plans d'eau artificiels, la densité larvaire a été réduite, passant à 38,9 %, 39,3 % et 65,1 % à des doses de 2 g/m2, 4 g/m2 et 8 g/m2 respectivement. Dans les rizières, à une dose de 2 kg/ha, la densité des larves immatures a été réduite, passant à 33,1 % et 28,6 %, sept jours après le traitement. Des recherches supplémentaires sont nécessaires pour expliquer la faible efficacité de ce larvicide.
1Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to H. Vatandoost:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
and M.R. Abai:
This e-mail address is being protected from spambots. You need JavaScript enabled to view it
).
2National Institute for Environmental Health Research, Tehran, Islamic Republic of Iran.
Received: 16/01/13; accepted: 04/06/13
EMHJ, 2014, 20(4): 229-235
Introduction
The control of immature stages of mosquitoes can be achieved through application of contact larvicides, insect growth regulators, surface films, stomach larvicides (including bacterial agents) and biological pathogens [1]. The microbial pathogens of mosquito larvae comprise viruses, bacteria, fungi, protozoa and nematodes [2]. Bacillus thuringiensis var israelensis (Bti) is a Gram-positive, aerobic, rod-shaped bacteria that naturally occurs in soil and on plants [3]. Bti forms spores during the stationary phase of its growth cycle and the dead spores produce a crystal protein that interferes with the digestive system of mosquito larvae [4]. Crystal toxins from Bti are ingested by mosquito larvae, and after solubilization and proteolytic cleavage, the activated toxin interacts with the midgut epithelium leading to death of the larvae [5]. Different strains of Bti produce different types of toxin which affects a narrow taxonomic group of insects [4].
In 1986, a bacterium isolated from dead larvae of Anopheles stephensi was recovered in the Iranian province of Lorestan, and the new-found bacterium was labelled B. thuringiensis M-H-14. Different species of Anopheles and Culex immature mosquitoes were shown to be sensitive to this toxic bacterium, which killed only its target without causing any damage to other wildlife [6]. The toxin works on a receptor in the gut that only Anopheles spp. possess, making B. thuringiensis M-H-14 completely innocuous to all other living species, including human beings. Within minutes of ingesting the toxin, the Anopheles spp. larva stops feeding as its cell walls swell and burst, causing death [7].
A local formulation of B. thuringiensis M-H-14 is marketed under the trade name Bioflash®. The granule formulation has heterogeneous particles similar to corn grit; when sprayed onto water the particles float to the surface and co-adhere for up to 3 weeks The active ingredient constitutes 10% of the formulated product, with a reported potency of 18 000 international toxic units (ITU)/mg. The manufacturer recommends a target dosage of 1.7 kg/hectare of the formulated product [8]. Due to some reports of its lack of efficacy in the field a study was designed to evaluate different formulations of Bioflash® in a variety of vector breeding sites in Kazerun, Fars Province. The efficacy and residual activity of Bioflash® granule 10% and wettable powder formulations were assessed using different methods and dosages against immature stages of Anopheles and Culex spp.
Methods
Study area
The selected sites were located on the southern slopes of the Zaghros mountains, where there is a sub-tropical climate. Simulated ponds were dug in the yard of Kazerun Medical Research Station (29°37´13˝ N; 51°38´30˝ E, elevation 832 m) in Kazerun city. The field trial was carried out at rice field of Bazarengan village (29°44´55˝ N; 51°33´10˝ E, elevation 773 m), which is 25 km north-west of Kazerun city (Figure 1).
Materials tested
Bioflash® granule 10% formulation (Nature Biotechnology Company) was applied for all the experiments carried out in the laboratory, aquarium, semi-field and field conditions. Each package weighed 85 g and was recommended by the manufacturer for application to 500 m2. The powder formulation was delivered from the Iranian Ministry of Health and Medical Education and used also for all laboratory, semi-field and field experiments. The manufacturer’s recommended dosage is 1.7 kg/ha, which involves doses for different areas being precisely weighed using an electronic digital balance and packed in foil sheets for application in the field. The packed granule or wettable powder formulations were uniformly scattered with a gloved hand at simulated ponds and rice plots.
Larval tests
The Beech strain of An. stephensi obtained from the insectary of the London School of Hygiene and Tropical Medicine was used for laboratory tests. The strain has been colonized at the insectary of the School of Public Health at Tehran University of Medical Sciences, Islamic Republic of Iran since July 2005. The Kazerun strain of An. stephensi, which had been colonized since 1960 in the insectary of Kazerun Medical Research Station in Kazerun, was used for aquarium trials. Due to the low density of anopheline larvae in the simulated ponds, the larvae were collected from rice plots in Bazarengan village and transferred in the same water to a room near the ponds and the predators and aquatic insects were removed. The larvae were distributed in batch of 150–200 to the simulated ponds. The species compositions at semi-field and field sites were determined based on 10% sampling using dipping methods. The larvae and pupae were reared in enamel pans covered with nets and the emerged adults anaesthetized with chloroform and pinned for identification.
Larval susceptibility test
The Beech strain of An. stephensi was used for biopotency activity and determination of lethal concentrations according to World Health Organization (WHO) recommended methods [9]. Late 3rd to early 4th instar larvae were selected and 25 larvae were transferred into 50 mL glass beakers. The required amount of Bioflash® granule 10% were added to 224 mL of dechlorinated tap water inside the 400 mL glass beakers. Mortality was recorded after 24 h. Moribund larvae (i.e. unable to reach the water surface on touch with a plastic fine rod) were considered as dead. The experiments were replicated at least 4 times. The tests of treated groups were rejected if the control mortality was higher than 20% or when pupation was 10% of tested larvae.
Bioassay in standard aquaria
Bioassays were done in 4 glass aquaria (3 treated groups and 1 control group) (dimensions of 33×100×70 cm) containing 130 L tap water. In order to discharge the water from the aquarium, a valve was set 15 cm from bottom of aquarium. The aquaria were placed in a room with windows allowing a natural photoperiod. Batches of 50 3rd instar larvae were transferred by means of screen loops to each aquarium 24 h before treatment. Four replicates and an equal number of untreated control groups were set up simultaneously for each test of both granular and powder formulations. The formulations were applied at 56.1 mg/130 L aquarium, equivalent to the recommended dosage of 1.7 kg/ha for Bioflash® granule 10%. Dead larvae (i.e. showing no sign of moving to the surface when the cervical region was probed with a fine plastic rod) were recorded 48 h after the treatment. Moribund larvae (i.e. incapable of rising to the surface) were counted and added to dead larvae for estimation of the percentage mortality. From the 3rd day after treatment, 10 L of water was discharged daily 5 times per week in the morning. A total of 50 L of tap water was added on the 5th day afternoon. The experiments were stopped when the mortality dropped to 70% according to the WHO method described for larvicides [9]. The percentage mortality was corrected for control mortality using Abbott’s formula [10].
Semi-field trials
The simulated ponds (100×100×50 cm) were dug at a distance of about 1 m apart and were fully lined with plastic sheeting and subsequently filled with tap water to a depth of 30–40 cm. The water level was maintained throughout the trial period. The ponds were left for 2 weeks until oviposition of mosquitoes occurred, and treatment of both granular and powder Bioflash® formulations were carried out when adequate numbers of 3rd and 4th instar larvae and pupae, whether Culex or Anopheles spp., were found in the ponds. Due to the low density of anopheline larvae in simulated ponds, the larvae were collected from rice field and transferred with the original water inside the plastic buckets. The collected larvae were distributed in enamelled pans, and the other aquatic insects including predators were isolated. The larvae were counted and added to the simulated ponds. The pre-treatment density of immature mosquitoes was monitored, and 3 pre-weighted aliquots of Bioflash® granule 10% or powder formulation at dosages of 2, 4 and 8 g per pond added to the water surface. Samples of 10 dips were taken at the corners, middle and middle of every side of the simulated pond and continued from the first day before treatment until 10 days post-treatment.
Field trials
In view of the importance of rice fields as mosquito breeding sites, suitable plots for the experiments were identified in Bazarengan village, 25 km of north-west of Kazerun city. The water source for the cultivation of rice was the Shapoor river (water level range 5–10 cm). There was a laminar flow of water at the plots. The rice plants were at vegetative stage and the height ranged 20–30 cm during the assessment of the products. Eight rice plots were selected for the experiments. Bioflash® was applied at 2 kg/ha ; the amount of product was calculated based on the area of each rice field and was spread with a gloved hand. The dipping for density estimation was made at the margins of rice fields using standard dippers and 1 dip made at every 1 m of margin. The immature larvae of every dip were poured into the enamelled pan and each instar larva and pupa was counted and recorded in the relevant form. The data were recorded before and after treatment for each experiment until 7 days post-treatment. The density of immature larvae was calculated as the mean number/10 dips. Species composition of immature mosquitoes was determined based on random sampling.
Data analysis
The data obtained from all replicates of bioassays were pooled separately for each experiment type. Median lethal concentration (LC50) and 90% lethal concentration (LC90) and confidence intervals were calculated using Probit software program, version 1989. The LC50 and LC90 values were chosen based on chi-squared values and degrees of freedom. If the control mortality rate was between 5% and 20%, the mortality rate in treated groups was corrected according to Abbott’s formula [10]. Tests with control mortality > 20% or pupation > 10% were discarded. The percentage reduction of the density was calculated using the Mulla’s formula separately for different instar larvae and pupae [11]. The reduction rates were transformed to arc sine values. Differences between treatments were compared by analysis of variance with treatment and number of days as independent factors, and differences between larval densities pre- and post-treatment were compared using Student t-test. Statistical analyses were done using SPSS, version 11.5.
Results
Species composition of immature mosquitoes
The following anopheline mosquitoes were collected and identified in the study: Anopheles dthali, An. superpictus, An. fluviatilis, An. stephensi, while the dominant species was An. dthali. From the culicine subfamily we collected Culex pipiens, Cx. tritaeniorhynchus, Cx. pseduvishnoai, Cx. quinquefasiatus, Cx. bitaeniorhynqus, Cx. theileri and Cx. sitiens. The greatest density was recorded for Cx. pipiens.
Laboratory trials
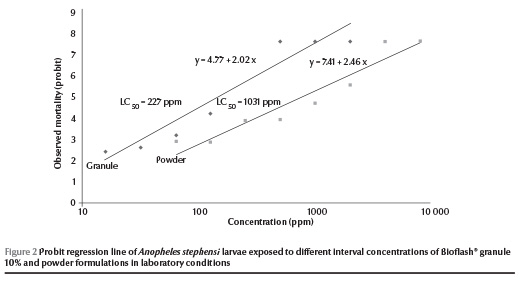
A 100% mortality of An. stephensi larvae was observed at 512 ppm of the Bioflash® granules at 24 h post-treatment. At lower concentrations (< 128 ppm) of granular formulation, the mortality rate of larvae ranged from 0% to 22.2%. The LC50 and LC90 values of the granular formulation were recorded as 227 and 977 ppm respectively. In comparison, the powder formulation showed 100% mortality at 4096 ppm at 24 h post-treatment and the LC50 and LC90 values were 1031 and 3422 ppm respectively (Table 1). Zero mortality was recorded among control larvae during these tests. The regression lines of mortality at different dosages of Bioflash® granules and powder formulations were plotted for An. stephensi (Figure 2).
Aquarium trials
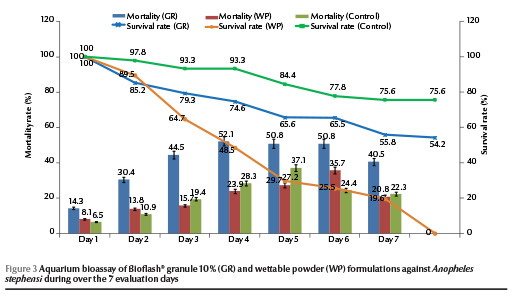
Application of BioFlash® at 56.1 mg per 130 L aquarium, equivalent to the recommended dosage of 1.7 kg/ha, resulted a less than 50% reduction in density of An. stephensi larvae on day 6 (range 14.3%–50.8% and 8.1%–35.7% for granule and powder formulations respectively) (Figure 3). There was no significant difference in bioefficacy of the formulations until day 6 and then the activity decreased to zero. The survival rates of the exposed larvae to the granule formulation were 65.6% and 54.2% on days 6 and 8 respectively.
Semi-field trials
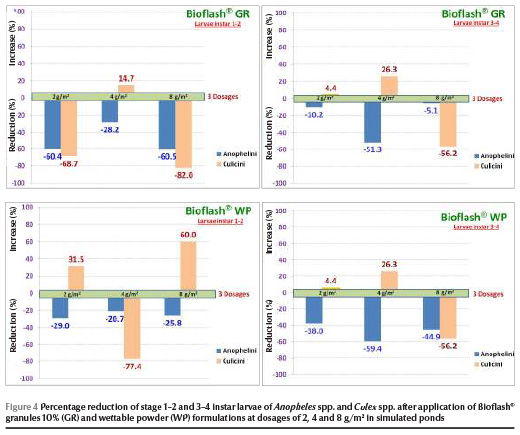
On day 9 of the semi-field trials Bioflash® granules 10% at dosages of 2, 4 and 8 g/m2 caused reductions of 28.2%, 60.4% and 60.5% respectively against stage 1–2 instar larvae and of 5.1%, 10.2% and 51.3% respectively against stage 3–4 instar larvae. Powder formulation showed reductions ranging from 20.7% to 29.0% and 38.0% to 44.9% against stage 1–2 and 3–4 instar larvae respectively for 9 days post-treatment (Figure 4).
Field trials
Bioflash® granule and powder formulations exhibited low efficacy at a single dosage (2 kg/ha) in rice fields. The percentage reductions in densities of immature anophelines were 37.7% and 37.8% for powder and granules respectively and for immature culicines were 37.2% and 49.6% respectively at 7 days post-treatment (Table 2).
Discussion
During recent decades, different commercial products of the bacterial control agent B. thuringiensis (e.g. Bactimos® and Tecknar®) have been imported to the Islamic Republic of Iran and evaluated in semi-field and field conditions [12,13]. The country is now producing B. thuringiensis M-H-14 on a commercial scale under the trade name Bioflash®. This product had been evaluated by different researchers in the country before commercial production. In this study, the larvicidal toxicity of Bioflash® granules 10% and powder formulation, manufactured by the local producer, were assessed against An. stephensi in the laboratory using WHO recommended methods [9]. The results showed that LC50 and LC90 values were 227 and 9767 ppm respectively. The same tests on powder formulation revealed LC50 and LC90 values of 1031 and 3422 ppm. However, with application of other formulations or products, very low LC50 values have been reported from Islamic Republic of Iran, such as 0.08, 0.5 and 2.6 ppm for different malaria vectors including An. stephensi, An. dthali and An. fluviatilis respectively [14]. In a laboratory study carried out in India on Bioflash® granules 10%, the LC50 value was calculated as 0.14 ppm against An. stephensi [7].
The WHO Pesticide Evaluation Scheme determined the ITU value of Bioflash® granule 10% by calculating and comparing its activity with that of IPS-82 (the international reference standard for Bti) at 15 000 ITU Ae. aegypti/mg and the LC50 and LC90 values were 0.01126 mg/L and 0.02218 mg/L respectively for IPS 82 and 1.66639 mg/L and 3.32804 mg/L respectively for Bioflash® granule 10% respectively [Adak T et al., unpublished report to the WHO Pesticide Evaluation Scheme, 2008]. The potency of Bioflash® granule 10% was equivalent to 191 ITU/mg, about 100 times lower than that reported by the manufacturer.
In this study, for the first time, the aquarium bioassay method was employed to determine the residual effects of a larvicide in the Islamic Republic of Iran. Although the bioassay showed a 50% reduction in larval density up to 7 days post-treatment when exposed to Bioflash® granule formulation, n similar tests a 96%–100% reduction was shown against Ae. aegypti larvae up to 94 days post-treatment [7].
In semi-field trials the percentage reductions of An. stephensi larvae were 28.2%–60.5% and 20.7%–44.9% at 3 dosages (2, 4 and 8 g/m2) of both formulations and, surprisingly, the highest dosage (8 g/m2) of granule formulation showed a lower reduction in larval density than the lowest dosage (2 g/m2). There were no significant differences in efficacy between the 2 formulations at any of the 3 doses. A similar trend of density reduction was also seen in the pupae and the culicine larvae following application of 3 dosages of both formulations.
A similar study was conducted in the Ghasserghand area of south-east Islamic Republic of Iran, where malaria is endemic. In order to determine the efficacy of Bioflash® granule 10% at 3 dosages, a semi-field trial was carried out in ponds lined with plastic sheeting. The percentage reductions of larval density for both Anopheles spp. and Culex spp. were < 50% during the post-treatment period at dosages of 0.85, 1.7 and 3.4 kg/ha, while the reductions were < 50% at 6.8 kg/ha and < 60% at 15 kg/ha. Even at 30 kg/ha, > 80% mortality was obtained only on day 5 and day 7 post-treatment, indicating that at this high dosage, the formulation was an effective control for a short period of only 3–4 days [Ladonni H, unpublished report to the WHO Pesticide Evaluation Scheme, 2004]. Another semi-field trial around Delhi, India showed a reduction rate of 30% 2 days after application of Bioflash® granules 10% at 1.36 g/m2 against An. subpictus in rainwater pools [Adak T et al., unpublished report to the WHO Pesticide Evaluation Scheme, 2008]. In the current study, the field trial of application of Bioflash® granules 10% at 2 kg/ha resulted in a moderate reduction of both Anopheles and Culex spp. larvae in rice plots, ranging between 37.7% and 49.6% respectively on day 9 post-treatment.
Conclusions
All the findings of this study indicated a low efficacy of Bioflash®, both granule and wettable powder formulations, at the recommended manufacturer’s dosage, as well as at 2 higher dosages of this product against anopheline and culicine larvae in laboratory, aquarium, semi-field and field conditions. These findings are consistent with various trials of the product at different parts of the world. Further follow-up investigations are required to establish whether the poor results are due to the formulations, storage or environmental conditions or to resistance of mosquito larvae to this product. Any modifications and improvements to the product by the manufacture need to be reassessed.
Acknowledgements
We thank Dr M. Ghooya, Head of the Iranian CDC for support during this study. We are grateful for the technical assistance of Mr G. Afrouzandeh and Mr N. Vadea, the field and laboratory staff of Kazerun Medical Research Station and Mr H. Salmanpour (driver). We also thank Mrs F. Rafi, School of Public Health, Tehran University of Medical Sciences, for her great efforts of mass colonization of the An. stephensi during the laboratory tests.
Funding: This study was funded by the Environmental Research Institute, Tehran University of Medical Sciences; grant no. 14134. This research was also supported in part by the Ministry of Health and Medical Education, Islamic Republic of Iran.
Competing interests: None declared.
References
- Malaria entomology and vector control. Tutors’ Guide. Geneva. World Health Organization, 2013 (http://apps/.who.int/iris/handle/10665/85890, accessed 23 November 2013)..
- Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Medical and Veterinary Entomology, 2007, 21(1):2–21.
- Ibrahim MA et al. Bacillus thuringiensis A genomics and proteomics perspective. Bioengineered Bugs, 2010, 1(1):31–50.
- Sanahuja G el al. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnology Journal, 2011, 9:283–300.
- Poopathi IS, Tyagi BK. Mosquitocidal toxins of spore forming bacteria: recent advancement. African Journal Biotechnology, 2004, 3(12):643–650.
- Report of the twelfth WHOPES working group meeting. Geneva, World Health Organization, WHO Pesticide Evaluation Scheme, 2009 (http://whqlibdoc. who.int /hq/2009/WHO_HTM_NTD_WHOPES_2009_1_eng.pdf, accessed 23 November 2013).
- Moazami N. Controlling malaria, the vampire of the technological age. A world of science. UNESCO Quarterly Newsletter, 2005, 3:15–19.
- Malaria entomology and vector control. Participant’s guide. Geneva World Health Organization, 2012 (http://apps.who.int/iris/handle/10665/85890, accessed 23 November 2013).
- Guidelines for laboratory and field testing of mosquito larvicides. Geneva, World Health Organization, 2005 (http://www.who.int/entity/whopes/resources /resources_2005/en, accessed 23 November 2013).
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 1925, 18:265–267.
- Mulla MS et al. Control of chironomid midges in recreational lakes. Journal of Economic Entomology, 1971, 64:301–307.
- Motabar M et al. Larvicidal activity of Bacillus thuringiensis H-14 (Teknar®) on mosquito larvae in rice fields, southern Iran. Iranian Journal of Public Health, 1986, 15(1-4):21–29.
- Kasiri H, Zaim M. Field assessment of Bacillus thuringiensis serotype H-14 (Bactimos® WP) , Abate® and fuel oil against Anopheles and Culex in south of Iran. Iranian Journal of Public Health, 1997, 26(3-4):69–76.
- Hanafi-Bojd AA et al. Susceptibility status of Anopheles dthali and An. fluviatilis to commonly used larvicides in an endemic focus of malaria, southern Iran. Iranian Journal of Arthropod Borne Diseases, 2006, 43:34–38.



